

External morphology of the adult of Stalachtis phlegia susanna (Fabricius, 1787) (Lepidoptera: Riodinidae)
Morfología externa del adulto de Stalachtis phlegia susanna (Fabricius, 1787) (Lepidoptera: Riodinidaee)
Morfologia externa do adulto de Stalachtis phlegia susanna (Fabricius, 1787) (Lepidoptera: Riodinidae)
External morphology of the adult of Stalachtis phlegia susanna (Fabricius, 1787) (Lepidoptera: Riodinidae)
SHILAP Revista de lepidopterología, vol. 44, núm. 176, 2016
Sociedad Hispano-Luso-Americana de Lepidopterología
Recepción: 09 Diciembre 2015
Aprobación: 08 Abril 2016
Publicación: 30 Diciembre 2016
Resumen: Se estudia aquí la completa morfología externa completa del adulto de Stalachtis phlegia susanna (Fabricius, 1787) con base en microscopía óptica y electrónica de barrido. Esta especie es muy abundante en la región del Bosque Atlántico del Estado de Sergipe, Brasil. Se trata del primer estudio de este tipo en Riodinidae neotropicales.
Palabras clave: Lepidoptera, Riodinidae, abdomen, cabeza, tórax, Mata Atlántica, Región Neotropical.
Abstract: The complete external morphology of the adults of Stalachtis phlegia susanna (Fabricius, 1787) is here studied based on optical and scanning electron microscopy. This species shows great abundance in the Atlantic Forest region of the state of Sergipe, Brazil. This is the first study of this kind concerning to Riodinidae in the Neotropics.
Keywords: Lepidoptera, Riodinidae, abdomen, head, thorax, Atlantic Forest, Neotropics.
Resumo: A morfologia externa completa do adulto de Stalachtis phlegia susanna (Fabricius, 1787) é aqui tratada com base em morfologia ótica e microscopia eletrônica de varredura. Tal espécie é muito abundante na região de Floresta Atlântica do estado de Sergipe, Brasil. Trata-se do primeiro estudo deste tipo para Riodinidae neotropicais.
Palavras-chave: Lepidoptera, Riodinidae, abdome, cabeça, tórax, Floresta Atlântica, Região Neotropical.
Introduction
The order Lepidoptera includes butterflies and moths, comprising holometabolic individuals with membranous wings and body covered with scales. About 160 thousand species have been described, but it is believed that there are about 500 thousand species throughout the world, which will possibly increase from faunal survey of fauna and other taxonomic studies (GASTON, 1991; KRISTENSEN et al., 2007; DUARTE et al., 2012).
Butterflies are diurnal individuals, being important environmental indicators because they have close relationships with their food resources, a high rate of reproduction, and can play important ecological roles through their herbivory and pollination relationships (FREITAS & MARINI- FILHO, 2011). According to the Neotropical butterflies Checklist (LAMAS, 2004), they are divided into two large superfamilies: Hesperioidea and Papilionoidea. In Brazil, the number of already record butterfly species reaches 3,288 (BROWN & FREITAS, 1999).
Riodinidae is a cosmopolitan family of butterflies, most widely distributed however, in the Neotropics, with about 1,300 species. It shows high diversity in the shape and colour of the wings, often having metallic appearance. Males have reduced forelegs with fusion of the tarsomeres, and a well developed uncus (SCOBLE, 1992; DUARTE et al., 2012).
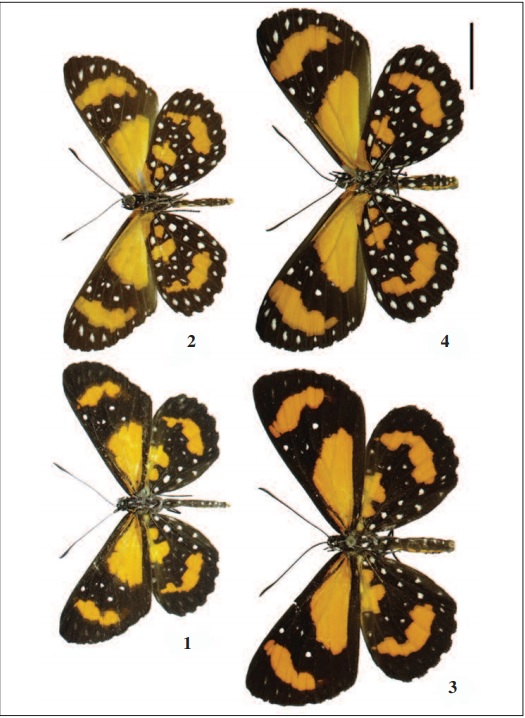
The family is not only notable for numbers of species, but also for phenotypic, morphological and ecological diversity (STICHEL, 1910-1911; CALLAGHAN, 1983; DEVRIES, 1990, 1991, 1997; D’ABRERA, 1994; HALL, 1999; HALL & HARVEY, 2002). Stalachtis phlegia susanna (Fabricius, 1787) (Figs 1-4) is a medium sized species common in the region, being easily found in Atlantic Forest areas in the state of Sergipe, and tropical and subtropical forests throughout southeastern Brazil. S. phlegia susanna is presumably to predators as suggested by its slow flight and bright orange colors (CALLAGHAN, 1986).
Morphological studies on Lepidoptera are of great importance in establishing the systematic relations of various groups (LEITE et al., 2010). Works dealing with the external morphology of head, thorax and abdomen were carried out in several families of butterflies (EHRLICH, 1958, 1960; SRIVASTAVA, 1961, 1962, 1965; MILLER, 1971; CASAGRANDE, 1979a,b,c; SORENSEN, 1980; BILOTTA, 1992, 1994a,b; BIZARRO et al., 2003a,b,c; DUARTE et al., 2001; MIELKE et al., 2004a,b,c; DUARTE, 2007; PALUCH et al., 2008; DIAS et al., 2010; LEITE et al., 2010a,b, 2011, 2013), but have not been carried out in Riodinidae, where studies on taxonomy and systematics deal only with venation and / or genitalia of both sexes (CALLAGHAN, 1997; HALL, 1999, 2001, 2003; DOLIBAINA et al., 2012) are recorded.
Material and Methods
Stalachtis phlegia susanna specimens of both sexes (n = 13 males and 9 females) were collected in the Atlantic Forest area belonging to the Parque Nacional Serra de Itabaiana - PARNASI (10º 40’S, 37º 25’W), located approximately 45 km from the capital Aracaju. The park is a significant remnant of the Atlantic Forest in the State of Sergipe, Brazil. The sampling was carried out actively with insect net, and individuals were sacrificed by thorax pressure. Some individuals were sacrificed with a killing bottle containing ethyl acetate, to preserve the thoracic structures.
For studies of venation, wings were removed from dry specimens and then cleared in Petri dishes, being first immersed in 70% ethanol (EtOH) and later in sodium hypochlorite (NaOCl) to complete the clearing process, afterwards were again submerged in 70% alcohol for neutralization and subsequently placed on absorbent paper to dry. For the structures of head, thorax, and abdomen, they were boiled in 10% potassium hydroxide (KOH) to soften the structures and facilitate the removal of scales.
The morphological drawings were made with a stereoscopic microscope coupled to a camera lucida. Further details of some structures were obtained using photographs from a scanning electron microscope (SEM) following standard procedures (Leite et al., 2010a).
The terminology applied follows mostly the latest works in the area (DIAS et al., 2010; LEITE et al., 2010a, b, 2011, 2013), though classical works were also consulted to better understand the morphological structures (SNODGRASS, 1935; MATSUDA, 1965; 1970; 1976).
Results
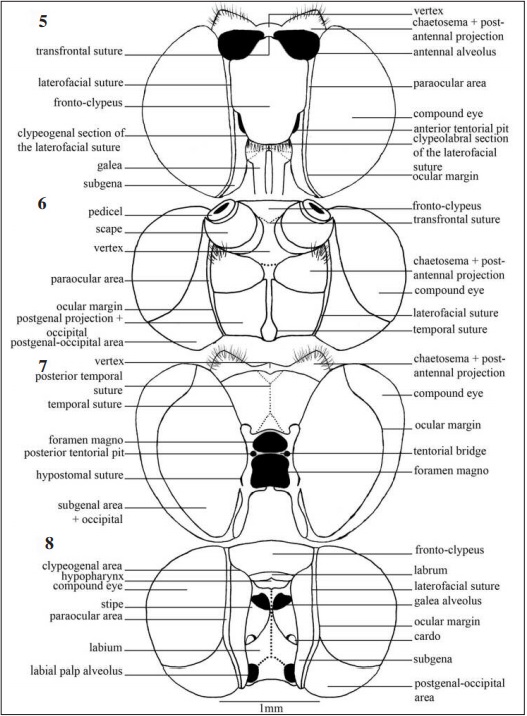
Hypognathous and sclerotized head, with compound eyes formed by numerous ommatidia, occupying the largest area, without ocelli and covered with numerous scales on its surface.
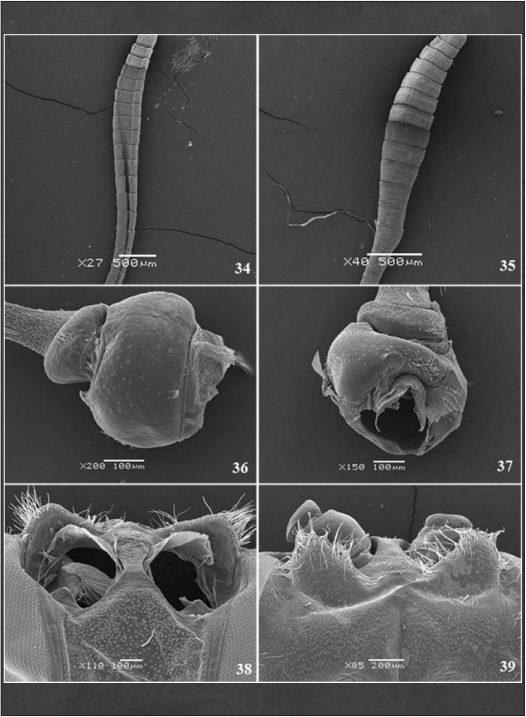
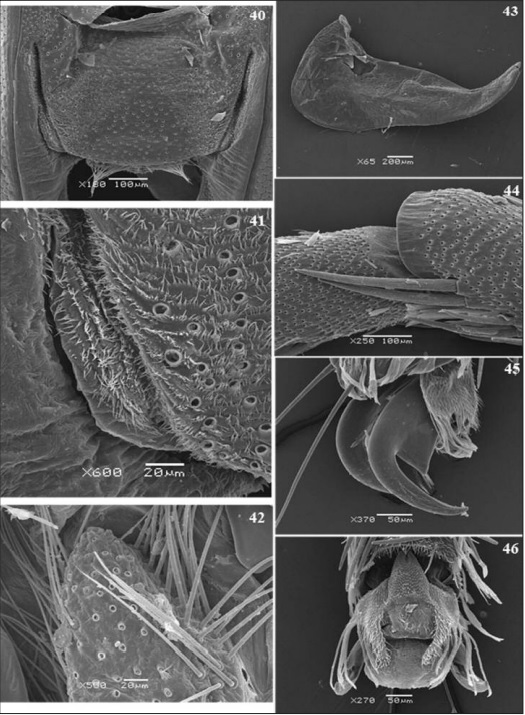
Frontal view (Fig. 5), post-antennal projections with many bristles and prominent chaetosema (Figs 38, 39), the projections form a semicircular vertex perpendicular to the transfrontal suture, limited laterally by the antennal alveolus which provide prominence to the suture. The fronto- clypeus is a prominent subrectangular plate bounded by the laterofacial suture, narrowing towards to the clypeogenal section of the laterofacial suture. Anterior tentorial pit (Figs 40, 41) located closely between the fronto-clypeus and subgena; compound eye globular limited by the ocular margin.
In dorsal view (Fig. 6) the compound eyes are limited anteriorly by the fronto-clypeus and posteriorly by the postgenal-occipital area which covers a significant part of the posterior portion. Post- antennal projections with triangular aspect on the anterior half and rounded on the posterior half.
The vertex in the posterior view (Fig. 7) is located anterior-medially on the head apex between the post-antennal projections and has under this, a trapezoidal shaped sclerite delimited laterally by the temporal suture and inferiorly by the upper margin of the foramen magnum. The subgenal region occupies the largest part of the posterior view, laterally limited by the compound eyes and dorso- medially by the temporal suture.
In ventral view (Fig. 8) the postgenal-occipital area lateroinferiorly located, limited superiorly by the compound eye and separated from the subgena by the hypostomal subgenal suture and the extension of the paraocular area respectively. Labium in the inferior medium portion as a subpentagonal structure.
In lateral view, the compound eye, wider than high, occupies the largest area of the head (Fig. 9) and is surrounded by the ocular margin. Postgenal-occipital area visible and representing one fourth of the head in lateral view.
CEPHALIC APPENDAGES (Figs 9-11, 34-37)
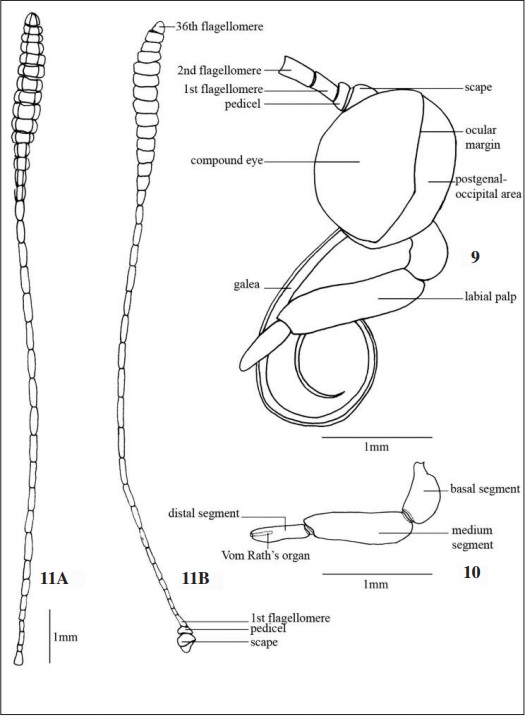
Mouthparts (Figs 9, 10): Galea three times larger than the width of the compound eye with no significant differences between the sexes. Tri-segmented labial palp. Basal segment slightly curved in the insertion region with the alveolus, median segment cylindrical and larger than the others, distal segment the shortest and narrow dorsoventrally with the presence of Vom Rath’s organ distally.
Antennae (Fig. 11A-B, 34-37): Clavate without carina, both sexes with 36-37 flagellomeres, 4.5 times longer than the width of the compound eye. No dimorphism. Dorsoventrally flattened pedicel with 1/3 of the scape size, presenting sensitive bristles (Figs 36, 37); scape rounded and robust. Short and rectangular flagellomeres increasing gradually from the 9th, acquiring a cylindrical shape until the 21st, then flat and tapered near the apex, thus providing the appearance of club.
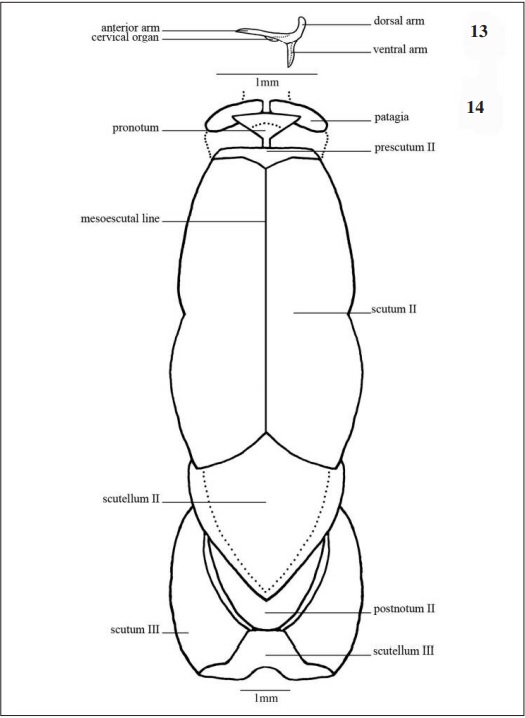
Cervical Region (Fig. 13): The most membranous region of the thorax. Cervical sclerite laterally in the middle of the pleural membrane, cervical organ at the posteroventral edge of anterior arm. Anteriorly articulated with the back of the head while posteriorly connected to the propleuron anteriorly to the prothorax.
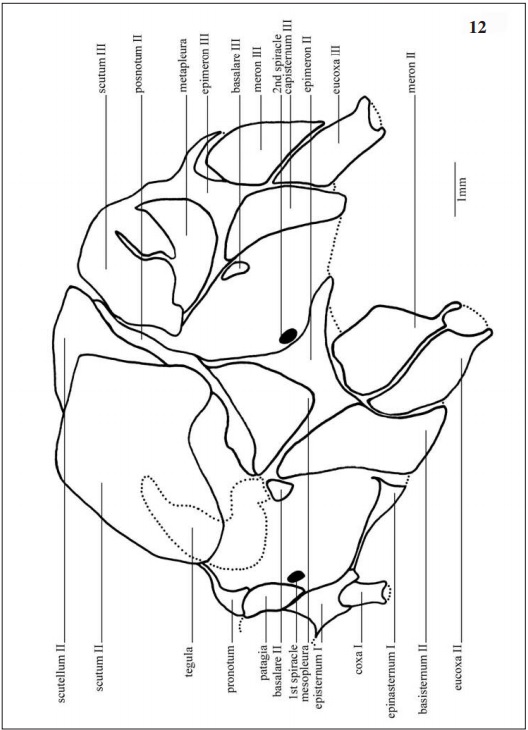
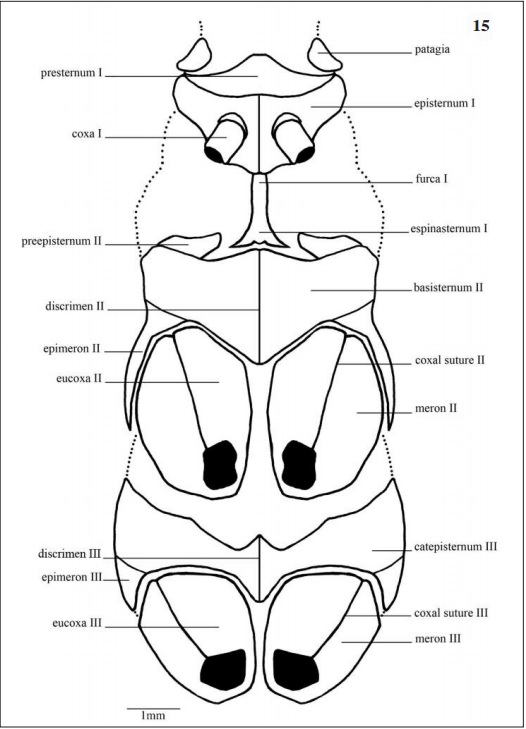
Prothorax (Figs 12, 14, 15): The smallest thorax division, with a pair of prothoracic legs and the most membranous area among the three segments. Laterally (Fig 12), the first spiracle, located posteroventrally to the patagia and dorsally to the episternum I; this one covers 1/3 of the prothorax region and articulates with the coxa I.
In dorsal view (Fig. 14) pronotum subtriangular, anterior margin flat and laterally connected to the patagia, posterior half narrow towards the anterior margin of the prescutum II.
In ventral view (Fig. 15) the episternum I occupies the largest area of the prothorax, limited anteriorly by the presternum, laterodistally by the patagia and posteromedially by the furca I. Alveolus coxal with cordiform shape.
Mesothorax (Figs 12, 14, 15): In lateral view (Fig 12) tegula (Figs 12, 20A-B) in the anteroventral portion of the scutum II, this one large and occupying half of the mesothorax area, limited posteriorly by scuto-scutellar suture II that separates the scutum II from the scutellum II, this last with an obtuse shape. Mesopleura surrounded posteriorly by the epimeron II and anteriorly by the capisternum II, this one subrectangular in shaped. Second spiracle posterior to the epimeron II.
In dorsal view (Fig. 14) anteriorly articulates with the prothorax through the prescutum II. Scutum II ovoid with smooth lateral invaginations, divided median-longitudinally by the mesoescutal line and posteriorly limited by the scutellum II, this one with losangular form.
Ventrally (Fig. 15), basisternum II anteriorly articulates with the preepisternum II and fuses lateroposteriorly with the epimeron II which surrounds the anterior half of the coxa II externally. Discrimen II median-longitudinally located separating the basisternum II.
Metathorax (Figs. 12, 14, 15): Laterally (Fig. 12) the scutum III occupies half of the segment. Basalare III and basalare III “pad” (Fig. 42) anterior-dorsally to the catepisternum III, this one subrectangular in shape. Meron III situated posteriorly to the catepisternum III with obtuse shape towards the eucoxa III.
In dorsal view (Fig. 14) the scutum III occupies most part of the segment as a pair of lateral plates, and is limited anteriorly by the scutellum II, medially by the postnotum II and posteriorly by the scutellum III.
In ventral view (Fig. 15) the catepisternum III anteriorly located, subrectangular, medially divided by the discrimen III, anteriorly concave and posteriorly surrounding the eucoxa III. The epimeron III internally surrounds the coxa III divided in meron III and eucoxa III separated by the coxal suture III.
THORACIC APPENDAGES (Figs 16-22, 43-46)
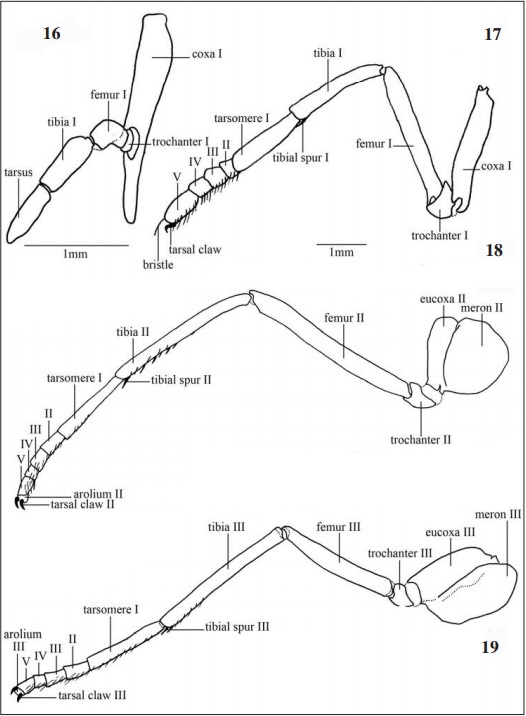
Legs (Figs. 16-19, 44-46): Riodinidae males (Figs. 1-2) have reduced prothoracic legs (Fig. 16), located in the anteroventral region of the prothorax. Coxa I, subrectangular, narrowing towards to the ventral extremity and larger than all the others structures; trochanter I has a flattened shape and articulates with femur I, this with cylindrical aspect and smooth dorsoventral curvature; tibia I, larger than the femur I, articulates with the tarsus, this as long as the tibia.
The females (Figs 3-4): have also reduced prothoracic leg (Fig. 17), presenting all the tarsomeres however. Robust coxa I that articulates with trochanter I in an obtuse form. Femur I, the largest among the other segments; tibia I provided with spurs (Fig. 44) located subapically and turned to the inner side. Tarsus formed by five tarsomeres, the proximal being the longest, the second to fourth smaller and quadrangular, the fifth slightly longer than the previous, all provided with spines on the ventral surface and bristles dorso-distally.
The mesothoracic leg (Fig. 18), similar in both sexes, is double the size of the prothoracic leg; rounded coxa II followed by trochanter II; cylindrical and subrectangular. Femur II articulates with tibia II, both elongated, tibia II covered with spines on the ventral surface and a pair of distal spurs; tarsus divided into five tarsomeres, the first being longer than the rest with distal and ventral spines, the second with 1/3 of the size of the first, third and fourth tarsomeres quadrangular and smaller than the second, developed arolium, pulvillus with bristles and a pair of claws distally. Similar to mesothoracic leg, the metathoracic leg (Fig. 19) differs by the femur, which in this case has equal length of tibia, this one without spines on the proximal third.
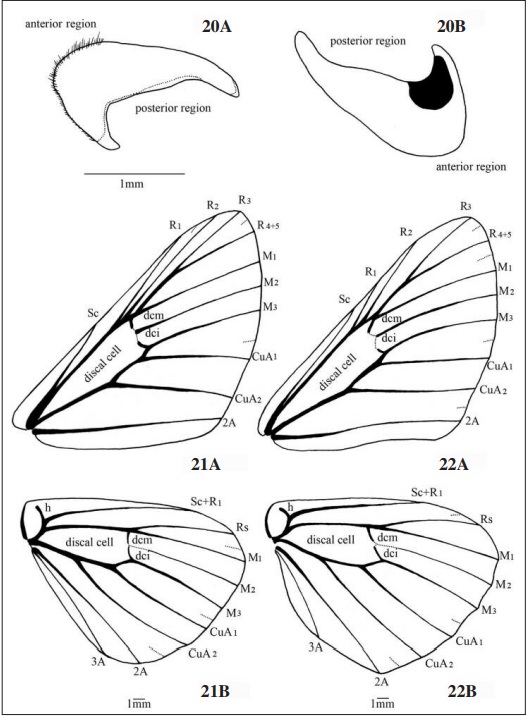
Wings (Figs. 21-22): Forewing (Figs. 21A, 22A) with subtriangular format for both sexes, with slight convexity in the outer margin. In females the subcostal vein (Sc) and the radial R1, R2 and R3 end in the costal margin, on males R3 reaches the apex of the wing which has a more pointed shape if compared with the females, R4 + 5 reaches the outer margin in both sexes. Discal cell elongated in females and slightly flattened on males with approximately half the length of the wing on both sexes, dci and dcm weakly connected with M2, dcs absent. M1, M2 and M3 in females have a slight curvature on the distal half when reaching the outer margin, in males however they are parallel; CuA1 and CuA2 extends horizontally and parallel in females and curved towards to the anal angle in males, the vein 2A presents rectilinear in males and sinuous in females.
Hindwings (Figs. 21B, 22B) with semi-oval shape, inner and outer margins lightly convex and without significant changes for both sexes. Humeral vein (h) with sharp curvature towards to the basal angle, Sc + R1 reaches the costal margin followed by Rs reaching the apex of the wing. Discal cell smaller if compared with the forewing; M1, M2 and M3 antagonistically curved to CuA1 and CuA2 towards the outer margin.
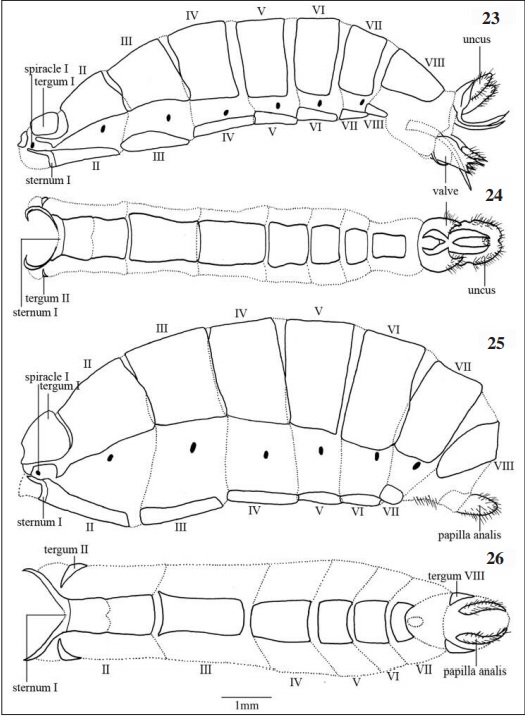
ABDOMEN (Figs 23-26)
Formed by ten segments, the last two in males and last three in females being modified in order to originate the genitalia structures; each segment has a dorsal tergum and ventral sternum well sclerotized and connected by a thin pleural membrane, where the spiracles are located. Both sexes have conspicuous changes in the first segment, both in tergum and sternum due to the connection with the metathorax.
Tergum I dorsally to the spiracle I, rounded shaped in males, subrectangular in females, smaller than the other segments for both sexes. Sternum I narrow, tapered and approaches the tergum II anterolaterally.
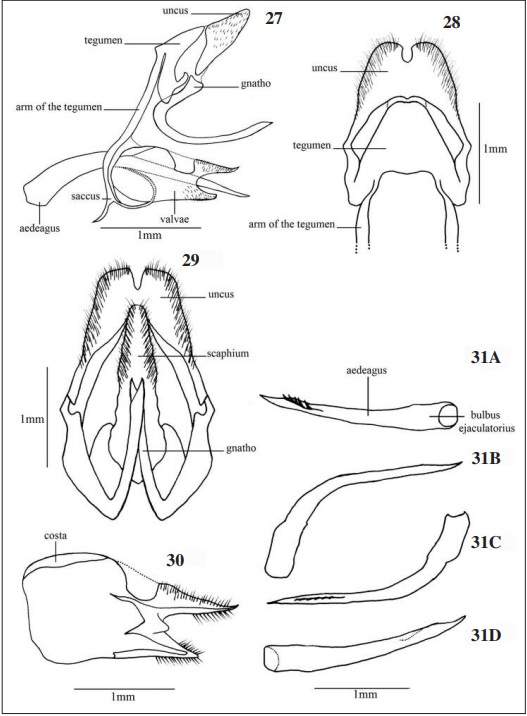
GENITALIA (Figs. 27-33)
Male genitalia (Figs 27-31): Uncus originated from tergum X, laterally with sub-oval format, located in the anterior region of the integument involving its end and covered by thick bristles. Tegumen formed by tergum IX, well sclerotized and limited posteriorly by the uncus. Gnatho hook shaped and dorsoposteriorly curved with a conspicuous medial curvature and a sharp curvature in the distal portion. Valvae subrectangular, medio-laterally flat and connected medio-posteriorly through internal projections. Aedeagus elongated, cylindrical in the anterior half and narrow distally where the vesicale is located.
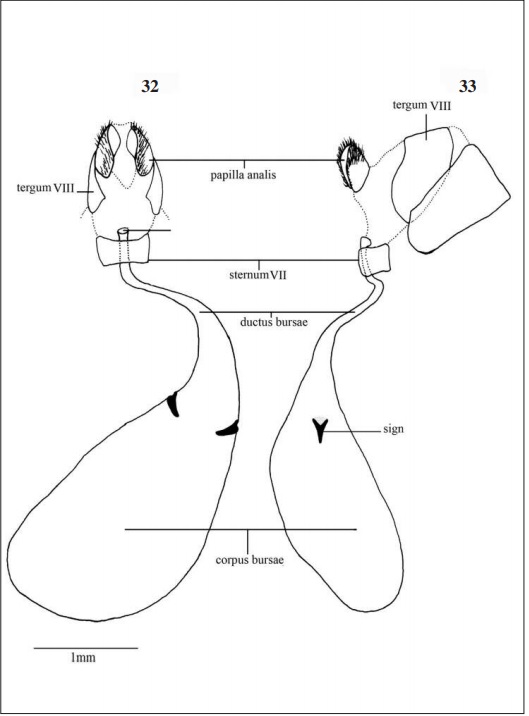
Female Genitalia (Figs 32-33): Consists of modifications of the last three segments of the abdomen. Tergum VIII dorsolaterally covers the genital region, IX and X form the papilla analis, suboval plates, very sclerotized and covered with bristles on the distal end. In ventral view the sternum VII is quite small and difficult to see; ductus bursae long with membranous aspect, it stretches gradually originating a translucent bag, the corpus bursae, saculiform and presenting two conspicuous “Y” shaped sings.
Discussion
Studies of complete external morphology are still scarce in the family Riodinidae.
Males and females do not exhibit structural differences in the cephalic region. Satalachtis phlegia susanna (Fabricius, 1787) presents some morphological aspects of the head similar to Glaucopsyche lygdamus (Doubleday, 1841) (Lycaenidae: Polyommatinae) in SORENSEN (1980) and Hemiargus hanno(Stoll, 1790) (Lycaenidae, Polyommatinae, Polyommatini) in DUARTE (2001).
Compound and globular eyes occupy more than 60% of head area, with a width exceeding the height, as opposed to what occurs in the superfamily Hesperioidea proposed by MILLLER (1971), no eye split forming two distinct areas as seen in (EHRLICH, 1960; CARNEIRO, 2012). Convex apex in frontal view, this one located between the postantennal projections, divergent from G. lygdamus and H. hannopresenting a flat vertex and postantennal projections invisible in frontal view.
S. phlegia susanna has a quadrangular fronto-clypeus, as also evidenced in Morpho helenor violaceus Fruhstorfer, 1912 (Nymphalidae), Iphimedeia hercules (Dalman, 1823) (Nymphalidae), Iphixibia anaxibia (Esper, 1801) (Nymphalidae ), Cytheritis portis thamyris (C. Felder & R. Felder, 1867) (Nymphalidae), Cytheritis aega (Hübner, 1822) (Nymphalidae), Pessonia epistrophus catenaria (Perry, 1811) (Nymphalidae), Grasseia menelaus nestira (Hübner, [1819]) (Nymphalidae) studied by BILOTTA (1992) and Heraclides anchisiades capys (Hübner, [1809]) (Papilionidae) LEITE (2010); transfrontal suture separating the frontoclypeal apex region also present in other Papilionoidea like Lycaenidae (SORENSEN, 1980;. DUARTE et al., 2001).
Posterior tentorial bridge similar to a horizontal bar that divides the two regions of the foramen magnum, followed by a subsequent tentorial pit; this differs from H. hanno where the tentorial bridge is reduced and foveae are located more ventrally.
The antennae have between 36-37 flagellomeres, without the presence of carina and sexual dimorphism strongly evidenced in other Papilionoidea such as Nymphalidae (BILOTTA, 1992) and Papilionidae (LEITE, 2010).
The labial palp of S. phlegia susanna has a similar morphology to Dachetola caligata(Stichel, 1911) (Riodinidae) (HALL, 2001) in which the median segment is more robust and twice larger than the others.
S. phlegia susanna has a narrower cervical sclerite when compared to H. hanno. Both males and females of S. phlegia susanna, have not significant differences in their thoracic structures and appendages, except the prothoracic leg that is reduced in males.
In dorsal view, the species presents a trapezoidal shaped pronotum, different from Lycaenidae as studied by SORENSEN (1980) and DUARTE (2007) where that same sclerite has a subtriangular format.
In lateral view of the metathorax it is possible to see the basilar III “pad” heavily covered with bristles, and located anterodorsally to the catepisternum III. The same was illustrated by DUARTE (2007) for H. hanno and Dynamine postverta (Cramer, 1779) (Nymphalidae) suggested by LEITE (2013).
The coxal ventral extension in the prothoracic legs of males, is similar to the genus Panara Doubleday, 1847 (Riodinidae) reviewed by CALLAGHAN (1997), Dachetola Hall, 2001 (Riodinidae) in HALL (2001), Pseudotinea Hall & Callaghan, 2003 (Riodinidae) studied by HALL et al. (2003).
Vein 3A is absent in the forewing of this species, it has been noted in some genera of Riodinidae (CALLAGHAN, 1997; HALL, 2001; HALL & CALLAGHAN, 2003) and in others groups of Papilionoidea (ERHLICH, 1958a; CASAGRANDE, 1979C; BILOTTA, 1994a; C. MIELKE et al., 2004b).
Tergum II presents projections on the anteroventral region, but there is no evidence of the presence of a prespiracular and postspiracular bar, diverging from the others Papilionoidea groups mentioned above.
Lateral membranous area between uncus and tegumen, a common feature in Riodinidae (CALLAGHAN, 1997; HALL, 1999; 2001; 2003; 2005; HALL & HARVEY, 2002; HALL & CALLAGHAN, 2003; PENZ & DEVRIES, 2006), is absent in S. p. susanna.
HALL (2003) showed the presence of pointed or rounded “teeth” at the end of the aedeagus in some specimens of the genus Pseudotinea, this characteristic is also observed in S. phlegia susanna which features five pointed “teeth” in the ventral region of the aedeagus, posterior to the vesica.
Agradecimientos
To the administrator of the Serra de Itabaiana National Park-PARNASI, for the research authorization in the area and for support. To the Foundation for the Support of Research and Innovation-FAPITEC/SE, for the financial support. To the Laboratório de Entomologia-LABENTO of the Universidade Federal de Sergipe (UFS) and the Laboratório de Estudos de Lepidoptera Neotropical of the Universidade Federal do Paraná, both for the support. To the Center of Electronic Microscopy (CEM) of the Universidade Federal do Paraná (UFPR) for microphotographs.
BIBLIOGRAPHY
BILOTTA, I., 1992.– Morfologia comparada da cabeça das espécies sulbrasileiras de Morphinae (Lepidoptera- Nymphalidae).– Revista Brasileira de Zoologia, .(3/4): 261-271.
BILOTTA, I., 1994a.– Morfologia comparada do tórax das espécies sulbrasileiras de Morphinae (Lepidoptera- Nymphalidae).– Revista Brasileira de Zoologia, 11(4): 691-713.
BILOTTA, I., 1994b.– Morfologia comparada do abdome das espécies sulbrasileiras de Morphinae (Lepidoptera- Nymphalidae).– Revista Brasileira de Zoologia, 11(4): 737-748.
BIZARRO, J. M. S; CASAGRANDE, M. M. & MIELKE, O. H. H., 2003a.– Morfologia externa de Thyridia psidii cetoides (Rosemberg & Talbot). I. Cabeça e apêndices (Lepidoptera, Nymphalidae, Ithomiinae).– Revista Brasileira de Zoologia, 20(2): 279-284.
BIZARRO, J. M. S; CASAGRANDE, M. M. & MIELKE, O. H. H., 2003b.– Morfologia externa de Thyridia psidii cetoides (Rosemberg & Talbot) II. Tórax e apêndices (Lepidoptera, Nymphalidae, Ithomiinae).– Revista Brasileira de Zoologia, 20(3): 419-425.
BIZARRO, J. M. S; CASAGRANDE, M. M. & MIELKE, O. H. H., 2003c.– Morfologia externa de Thyridia psidii cetoides (Rosenberg & Talbot) (Lepidoptera, Nymphalidae, Ithomiinae). III. Abdome e apêndices.– Revista Brasileira de Zoologia, 20(4): 681-684.
BROWN, K. S. & FREITAS., A. V. L., 1999. Lepidoptera.– In C. R. F. BRANDÃO, E. M. CANCELLO.– Biodiversidade do Estado de São Paulo, Brasil.– Invertebrados Terrestres: 225-245. São Paulo, FAPESP.
CALLAGHAN, C. J., 1983.– A study of isolating mechanisms among Neotropical butterflies of the subfamily Riodininae.– Journal of Research on the Lepidoptera, 21: 159-176.
CALLAGHAN, C. J., 1997.– A review of the genus Panara Doubleday, 1847 (Riodinidae) in southeast Brazil, with a description of two new subspecies.– Journal of Research on the Lepidoptera, 34: 21-38.
CALLAGHAN, C. J. & LAMAS, G., 2004. Riodinidae: 141-170.– In G. LAMAS (Ed.).– Checklist: Part 4A. Hesperioidea-Papilionoidea.– In J. B. HEPPNER (Ed.). Atlas of Neotropical Lepidoptera. Volume 5A: XXXIV + 439 pp. Association for Tropical Lepidoptera, Scientific Publishers. Gainesville.
CARNEIRO, E; MIELKE, O. H. H. & CASAGRANDE, M. M., 2012.– Head morphology of some Neotropical Hesperiidae (Lepidoptera).– Zootaxa, 3198: 1-28.
CASAGRANDE, M. M., 1979a.– Sobre Caligo beltrao (Illiger).– Morfologia externa da cabeça do adulto (Lepidoptera, Satyridae, Brassolinae).– Revista Brasileira de Biologia, 39(1): 223-227.
CASAGRANDE, M. M., 1979b.– Sobre Caligo beltrao (Illiger).– Morfologia externa do adulto-tórax. (Lepidoptera, Satyridae, Brassolinae).– Revista Brasileira de Biologia, 39(2): 347–355.
CASAGRANDE, M. M., 1979c.– Sobre Caligo beltrao (Illiger).– Morfologia externa do adulto- abdome. (Lepidoptera, Satyridae, Brassolinae).– Revista Brasileira de Biologia, 39(3): 711-716.
D’ABRERA, B., 1994.– Butterflies of the Neotropical region. Part VI. Riodinidae: IX + 880-1096 pp. Hill House, Victoria.
DEVRIES, P. J., 1990.– Enhancement of symbioses between butterfly caterpillars and ants by vibrational communication.– Science, 248: 1104-1106.
DEVRIES, P. J., 1991.– Call production by myrmecophilous riodinid and lycaenid butterfly caterpillars (Lepidoptera): morphological, acoustical, functional and evolutionary patterns.– American Museum Novitates, 3025: 1-23.
DEVRIES, P. J., 1997.– The butterflies of Costa Rica and their natural history. II. Riodinidae, 62: 343-364. Princeton Univ. Press, Princeton.
DEVRIES, P. J., 2000.– Entomophagy, Behavior, and Elongated Thoracic Legs in the Myrmecophilous Neotropical Butterfly Alesa amesis (Riodinidae).– Biotropica, 32(4a): 712-721.
DIAS, F. M. S., CASAGRANDE, M. M. & MIELKE, O. H. H., 2010.– Morfologia do exoesqueleto de adultos de Memphis moruus stheno (Pritwittz) (Lepidoptera, Nymphalidae, Charaxinae).– Revista Brasileira de Entomologia, 54(3): 376-398.
DOLIBAINA, D. R., LEITE, L. A. R., DIAS, F. M. S., MIELKE, O. H. H. & CASAGRANDE, M. M., 2012.– An annotated list of Symmachia Hübner, [1819] (Lepidoptera: Riodinidae: Symmachiini) from Parque Nacional da Serra do Divisor, Acre, Brazil, with the description of a new species.– Insecta Mundi, 249: 1-11.
DUARTE, M., Casagrande, M. M. & Mielke, O. H. H., 2001.– Morfologia externa do adulto de Hemiargus hanno (Stoll) (Lepidoptera, Lycaenidae, Polyommatinae, Polyommatini). I. Cabeça.– Revista Brasileira de Zoologia, 18(1): 225-238.
DUARTE, M., 2007.– Morfologia externa do adulto de Hemiargus hanno (Stoll) (Lepidoptera, Lycaenidae, Polyommatinae, Polymmatini) II. Região cervical, tórax e abdome.– Iheringia, Série Zoologia, 97(2): 194- 206.
EHRLICH, P. R., 1958.– The integumental anatomy of the monarch butterfly Danaus plexippus L., (Lepidoptera: Danaidae).– The University of Kansas Science Bulletin, 38: 1315-1349.
EHRLICH, P. R., 1960.– The integumental anatomy of the silver-spotted skipper, Epargyreus clarus Cramer (Lepidoptera-Hesperiidae).– Microentomology, 24: 1-23.
FREITAS, A. V. L & MARINI-FILHO, O. J., 2011.– Plano de Ação Nacional para Conservação dos Lepidópteros Ameaçados de Extinção: 124 pp. ICMBio, Brasília,
GASTON, K. J., 1991.– The magnitude of global insect species richness.– Conservation Biology, .: 283-296.
HALL, J. P. W., 1999.– A revision of the genus Theope its systematics and biology (Lepidoptera: Riondinidae: Nymphidiini): 127 pp. Scientific Publishers, Gainesville.
HALL, J. P. W. 2001.– A revision of the new riodinid butterfly genus Dachetola (Lepidoptera: Riodinidae).– Journal of the New York Entomological Society, 109(2): 183-195.
HALL, J. P. W. & HARVEY, D. J., 2002.– The phylogeography of Amazonia revisited: new evidence from riodinid butterflies.– Evolution, 56(7): 1489.
HALL, J. P. W. & CALLAGHAN, C. J., 2003.– A revision of the new riodinid butterfly genus Pseudotinea (Lepidoptera: Riodinidae).– Journal of Natural History, 37: 821-837.
HALL, J. P. W., 2003.– Phylogenetic reassessment of the five forewing radial-veined tribes of Riodininae (Lepidoptera: Riodinidae).– Systematic Entomology, 28: 23-37.
KAMINSKI, L. A; DELL’ERBA, R. & MOREIRA, G. R. P., 2008.– Morfologia Externa dos Estágios Imaturos de Heliconíneos Neotropicais: VI. Dione moneta moneta Hübner (Lepidoptera, Nymphalidae, Heliconiinae).– Revista Brasileira de Entomologia, 52: 13-23.
KRISTENSEN, N. P., SCOBLE, M. J. & KARSHOLT, O., 2007.– Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity.– Zootaxa, 1668: 699-747.
LEITE, L. A. R., CASAGRANDE, M. M. & MIELKE, O. H. H., 2010a.– External Morphology of the Adult of Heraclides anchisiades capys (Hübner, [1809]) (Lepidoptera-Papilionidae). I. Head, cephalic appendages and cervical region.– Brazilian Archives of Biology and Technology, 53(5): 1119-1126.
LEITE, L. A. R., CASAGRANDE, M. M. & MIELKE, O. H. H., 2010b.– External Morphology of the Adult of Heraclides anchisiades capys (Hübner, [1809]) (Lepidoptera-Papilionidae). II. Thorax and thoracic appendages.– Brazilian Archives of Biology and Technology, 53(6): 1407-1416.
LEITE, L. A. R., CASAGRANDE, M. M. & MIELKE, O. H. H., 2011.– External Morphology of the Adult of Heraclides anchisiades capys (Hübner, [1809]) (Lepidoptera-Papilionidae). III. Abdomen.– Brazilian Archives of Biology and Technology, 54(2): 331-336.
LEITE, L. A. R., CASAGRANDE, M. M. & MIELKE, O. H. H., 2013.– External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae.– Revista Brasileira de Entomologia, 57(2): 133- 148.
MATSUDA, R., 1965.– Morphology and evolution of the insect head.– Memoirs of the American Entomological Institute, .: 1-334.
MATSUDA, R., 1970.– Morphology and Evolution of the Insect Thorax.– Memoirs of the Entomological Society of Canada, 76: 1-431.
MATSUDA, R., 1976.– Morphology and Evolution of the Insect Abdomen: VIII + 534 pp. Pergamon Press, Oxford.
MIELKE, C. G. C., CASAGRANDE, M. M. & MIELKE, O. H. H., 2004a.– Estudo comparado de morfologia externa de Zaretis itys itylus (Westwood) e Agrias claudina annetta (Gray) (Lepidoptera, Nymphalidae, Charaxinae). I. Cabeça, apêndices e região cervical.– Revista Brasileira de Zoologia, 21(2): 357-369.
MIELKE, C. G. C; MIELKE, O. H. H. & CASAGRANDE, M. M., 2004b.– Estudo comparado da morfologia externa de Zaretis itys itylus (Westwood) e Agrias claudina annetta (Gray) (Lepidoptera, Nymphalidae, Charaxinae). II. Tórax e apêndices.– Revista Brasileira de Zoologia, 21(3) 421-433.
MIELKE, C. G. C; O. H. H. Mielke & M. M. Casagrande. 2004c.– Estudo comparado de morfologia externa de Zaretis itys itylus (Westwood) e Agrias claudina annetta (Gray). (Lepidoptera, Nymphalidae, Charaxinae) III. Abdome.– Revista Brasileira de Zoologia, 21(4): 905-912.
MILLER, J. Y., 1971.– The head capsule of selected Hesperioidea.– Journal of Research on the Lepidoptera, .(4): 193-214.
PALUCH, M., CASAGRANDE, M. M. & MIELKE, O. H. H., 2008.– Morfologia externa do adulto de Actinote melanisans (Lepidoptera: Heliconiinae: Acraeini).– Revista Brasileira de Zoologia, 25(3): 456-478.
PENZ. C. M. & DEVRIES, P. J., 1999.– Preliminary Assessment of the Tribe Lemoniini (Lepidoptera: Riodinidae) Based on Adult Morphology.– American Museum Novitates, 3284: 1-32, 16 figs.
SCOBLE, M. J., 1992.– The Lepidoptera: form, function and diversity. Natural History Museum Publications: 404 pp. Oxford University Press, Oxford.
SNODGRASS, R. E., 1935.– Principles of insect morphology: 667 pp. McGraw-Hill Book Company. New York and London.
SORENSEN, J. T., 1980.– An integumental anatomy for the butterfly Glaucopsyche lygdamus (Lepidoptera: Lycaenidae): a morphological terminology and homology.– Zoological Journal of the Linnean Society, 70: 55- 101.
SRIVSATAVA, K. P., 1961.– Studies on the lemon butterfly Papilio demoleus L.; (Lepidoptera). II Skeleto muscular mechanism (cervix and prothorax).– Indian Journal of Entomology, 23: 202-213.
SRIVASTAVA, K. P., 1962.– Studies on the lemon butterfly Papilio demoleus L. (Lepidoptera). III Skeleto muscular mechanism (pterothorax and its legs).– Indian Journal of Entomology, 24: 114-134.
SRIVASTAVA, K. P., 1965.– Studies on the lemon butterfly Papilio demoleus L. (Lepidoptera). Part V. Skeleto muscular system of the abdomen.– Zoologischer Anzeiger, 177: 217-236.
STICHEL, H. F. E. J., 1910-1911.– Family Riodinidae. Allgemeines. Subfamily Riodininae.– Genera Insectorum, 112: 1-452