

Host plant selection in Plebejus argus (Linnaeus, 1758) and its mutualistic ant. The role of plant architecture (Lepidoptera: Lycaenidae)
Host plant selection in Plebejus argus (Linnaeus, 1758) and its mutualistic ant. The role of plant architecture (Lepidoptera: Lycaenidae)
SHILAP Revista de lepidopterología, vol. 44, núm. 174, 2016
Sociedad Hispano-Luso-Americana de Lepidopterología
Recepción: 02 Febrero 2015
Aprobación: 08 Julio 2015
Abstract: In the Doñana National Park the larvae of Plebejus argus (Linnaeus, 1758) feed on Halimium halimifolium L. Willk. and maintain a mutualistic relationship with the ant Lasius niger (Linnaeus, 1758), nesting on the base of these shrubs. The architecture of this plant has been analysed trying to assess if there are visual and structural characters selected by both the associated ants and butterflies for egg laying that enable a quick recognition of those with a greater probability of occupancy. We also analyse whether the behaviour of selection of ants matches in areas of high and low density of nests. The results show that ants choose higher and more isolated plants both in areas of high and low density of nests. The selection of the butterfly for egg laying partially matches that of the ant, although isolation of the plant has no significance for butterflies. It is possible to identify egg-carrying target plants prior to scrub clearings, trying to mitigate the collateral effects that this management measure has on these species.
Keywords: Lycaenidae, plant architecture, habitat selection, Plebejus argus, Lasius niger, management, mutualism, egg laying, Spain.
Resumen: En el Parque Nacional de Doñana las larvas de Plebejus argus (Linnaeus, 1758) se alimentan en Halimium halimifolium L. Willk. y mantienen una relación mutualista con la hormiga Lasius niger (Linnaeus, 1758), que anida en la base de estos matorrales. La arquitectura de esta planta ha sido analizada tratando de evaluar si hay caracteres visuales y estructurales seleccionados tanto por las hormigas asociadas como por las mariposas para la puesta de huevos que permitan un rápido reconocimiento de los que tienen mayor probabilidad de ocupación. También analizamos si el comportamiento de selección de hormigas coincide en zonas de alta y baja densidad de sus hormigueros. Los resultados muestran que las hormigas eligen las plantas más grandes y más aisladas, tanto en las zonas de alta y baja densidad de hormigueros. La selección de la mariposa para la puesta de huevos coincide parcialmente con la de la hormiga, aunque el aislamiento de la planta no tiene ningún significado para las mariposas. Es posible así identificar plantas con cargas de huevos antes de los tratamientos de aclarado de matorral, para tratar de mitigar los efectos colaterales que esta medida de gestión tiene sobre estas dos especies.
Palabras clave: Lepidoptera, Lycaenidae, arquitectura de plantas, selección de hábitat, Plebejus argus, Lasius niger, manejo, mutualismo, puesta de huevos.
Introduction
In the Doñana National Park (SW Spain) Halimium halimifolium L. Willk. (known as “jaguarzo”) is the most abundant scrub species. This species supports the mutualistic relationship established between the butterfly Plebejus argus (Linnaeus, 1758) and the ant Lasius niger (Linnaeus, 1758), since on one hand it is the main foodplant for the butterfly larvae and on the other it is the physical support necessary for the development of ant nests in this landscape of sandy soils (RODRÍGUEZ et al., 1991, JORDANO et al., 1992). P. argus is a univoltine butterfly that is on the wing from late May to early July. In this protected area (DNP) this butterfly is the most abundant species (FERNÁNDEZ-HAEGER et al., 1976) and is present in most of the 1 km2 grids of the 540 km2 of the DNP. Its life history is closely linked to that of L. niger ants in a typical forced mutualism (RODRÍGUEZ et al., 1991;JORDANO et al., 1992). The butterfly larvae needs the protection of the ants and, like many other Lycaenidae, has a sugar producing mechanism in reward. Similarly L. niger has a wide distribution inside the park (GUTIÉRREZ et al., 2005).
The role that egg laying plays in the relationship between plants and insects is basic to understand the ecology and evolution of insects (THOMPSON & PELLMYR, 1991;AGOSTA, 2006). Insect females have developed specific behaviour for ovipositing in locations that are favourable for larval development and their expected survival, thereby increasing their realised fecundity (THOMPSON & PELLMYR, 1991;AWMACK & LEATHER, 2002). In the case of butterflies, host plant selection ranges from plant species to an individual plant within a population, or even specific parts of an individual (HOLEC et al., 2006;THOMPSON & PELLMYR, 1991).
Butterflies usually have low mobility larvae and therefore the development of their immature stages depends closely on where females lay their eggs (WAGNER & KARINA, 1997). Although there are errors in the selection of suitable substrates for egg laying, females usually discriminate where to lay the eggs (BROWERS & STAMP, 1987;CLARK & DENNETY, 1988). In batch laying species clutch size determines the importance of a correct choice (COURTNEY, 1984). It can be especially important in species with large clutch size, as success will depend on one or a few decisions, whereas in individual egg laying the female will take multiple decisions and the consequences of a mistake are diluted between the hits. However, in the latter case the female has to develop a behaviour that allows recognising repeatedly the best places for egg laying and survival of offspring.
The selection of the most suitable places is a complex process with a series of steps driven by multiple signals. In a first pre-alighting phase females must find and recognise the host plant following visual or olfactory cues. Later, in a post alighting phase females should evaluate the suitability of the plant to support their progeny. In this phase, females use visual, olfactory, gustatory or tactile cues. Among the visual stimuli, color, shape and height of the plant or branches are crucial, but acoustic communication between ants and imagos can’t be ruled out (DENNIS, 1983, 1985;ALVAREZ et al., 2005;BEYER & SCHULTZ, 2010;PRADEL & FISHER, 2011). Other stimuli have a chemical origin (REUDLER TALSMA et al., 2008;SUWARNO et al., 2010), respond to nutrition, palatability or plant age and phenology (RABASA et al., 2004;DOAK et al., 2006;SUWARNO et al., 2010;PATRICELLI et al., 2011). Sometimes egg laying spatially responds to the location of plants, as in the use of ephemeral herbaceous species, plants near nectar sources or connectivity between occupied patches (COURTNEY & COURTNEY, 1982; LÖRTSCHER et al., 1995;JANSEN et al., 2012). This process also addresses inter- or intraspecific antagonistic interactions, such as avoiding competition that sometimes leads to cannibalism among caterpillars and avoiding predators or parasitoids (SCHOONHOVEN et al., 1990;BARROS & ZUCOLOTO, 2005;ARAÚJO et al., 2006;RIIHIMÄKI et al., 2006;OBREGÓN et al., 2011).
In addition, the architectural form and complexity of the plant appears to be decisive in species with different trophic relationships (RIIHIMÄKI et al., 2006;RUDGERS & WHITNEY, 2006), and its influence can be key by facilitating the emergence of different temperature and humidity microhabitats in a plant (GONÇALVES-ALVIM & FERNANDES, 2001;BEYER & SCHULTZ, 2010). On the other hand, individual plants with a complex structure may facilitate the development of large herbivore populations and, therefore, reduce the probability of extinction, and the maintenance of a diverse invertebrate community (ARAÚJO et al., 2006;REID & HOCHULI, 2007).
Many species of Lycaenidae use ants as a signal to oviposit, especially in forced associations (PIERCE & ELGAR, 1985, JORDANO et al., 1992;WYNHOFF et al., 2008; PATRICELLI et al., 2011;JANSEN et al., 2012) but respond more weakly to them when the relationship is facultative (PIERCE & EASTEL, 1986). In any case, if the butterflies use the ants as a signal, (THOMAS et al., 2009) ant colonies have to be detectable by butterflies and be persistent in space and time.
In the case of ants the appropriate selection of the plant or foraging area may be critical to the success and survival of the colony itself (CAMARGO et al., 2003). Habitat selection that ants do may depend on the trunk diameter of the plant, the presence of aphids (PIERCE & ELGAR, 1985;BRISTOW, 1991;SAKATA & HASHIMOTO, 2000) or inter- and intra-specific competitive relationships among others factors (BOOMSMA et al., 1982;FOURCASSIÉ et al., 2012) and in some specific cases on other physical factors like flood regime (BOOMSMA et al., 1982;GUTIÉRREZ et al., 2005; HOLEC et al., 2006) or microclimate (THOMAS et al., 2009). Intraspecific competition for nesting sites seems to be relevant in Lasius species, since colonies have fixed nesting sites (BOOMSMA et al., 1982).
This relationship is based on the presence of the cistaceae, H. halimifolium a widespread shrub species in the DNP, which is the main foodplant of the butterfly larvae and the physical support necessary for the development of ant nests in a landscape of a sandy nature (RODRÍGUEZ et al., 1991,JORDANO et al., 1992).
In this paper we try to analyse the selection of plants that both ants and butterflies practice and specifically if there are visual cues of the plant that allow us to classify it as occupied or not. In the case of ants we first analyse selection of plants according to their appearance in areas with high density of ant nests. We then try to check if the detected pattern is consistent in areas with low densities of ant nests. We hypothesize a large discrimination in the selection of plants by their morphology in sites where the resource abundance is high, i.e. in places with high availability of plants. Conversely, we also hypothesize that discrimination in plant morphology will be lower in sites where the resource (low plant availability) is limited. For butterflies our model focuses only on areas of high density of ant nests since butterfly egg counting in low density areas did not yield sufficient data for reliable analysis due to the concomitant low abundance of butterflies.
Material and methods
The Doñana National Park (DNP) is located on the southwestern Atlantic coast of the Iberian Peninsula. Much of its surface is covered by scrub colonising former coastal sand dunes (GARCÍANOVO et al., 1977). The moisture content of the soil and resulting vegetation depends primarily on the height of the dunes over the underlying water table.
We have first analysed the relationship between ants, butterflies and plants in areas with previously known high density of ants and butterflies, where the density of butterfly eggs is enough to allow a reliable assay. During July, when the flying period of this univoltine butterfly is over and eggs are recently laid, we measured 202 plants along two 50 m length transects in two high density places 6 km apart (local scale).
Subsequently, we analysed ant preferences throughout all the DNP, differentiating between areas of high and low density of ant nests. For this purpose we randomly chose 50 squares of 1 ha from all over the park (landscape scale). Each square was subdivided into four equal subsquares and in each three lines of 10 m length were set up (thus twelve 10 m lines for each 1 ha square) (see GUTIÉRREZ et al., 2005). We measured a maximum of 10 H. halimifolium individuals per line (120 plants per frame). In cases of low density of plants in the square at least 60 plants were randomly chosen.
We checked a total of 4365 plants all over the DNP, 202 of them being in the high-density of ants and butterflies transect.
Three measures (in cm) of the aerial parts of the plant were recorded from each individual of H. halimifolium: the height, width (D1_halim) and maximum measurement perpendicular to it (D2_halim). We also measure the diameter of the stem at the base (D_base). The basal diameter is an easy measure to take in young plants, but not in older ones, since their stem may be creeping or branched. In these cases the whole surface in contact with the sand floor was measured, as an approximation to the surface used by ants to build their nest or butterfly larvae to hide during the day or where they finally pupate. An index of shape was calculated by dividing the height and width.
We also scored the presence of both Plebejus argus eggs and Lasius niger workers, as well as the degree of isolation of the plant based on the percentage of contact between aerial parts of neighbouring plants (isolation categories: Class 0 (0%), Class 1 (< 25%), Class 2 (25-50%), Class 3 (50-75%) and Class 4 (> 75%).
The relationship between plant variables was analysed by Pearson r correlation test eliminating those with r > 0.7. At the local scale, nonparametric tests were used on those variables that could not be normalised (SOKAL & ROHLF, 1984), i.e. degree of isolation or shape, and ANOVA in the remaining ones. The basal diameter was square root transformed to fulfil the assumptions of the analysis. In the detailed analysis of oviposition preferences we conducted a comparison of variance of these morphological variables, considering as factors both the presence of eggs and of ants (2- factor ANOVA).
At landscape scale each 1 ha square was first classified as low or high density, according to the median of ant nest abundance. Later, both the category of ant density and the presence itself were considered as factors in the analysis of preference within a multifactorial analysis of variance with plant height, D2_halim and the square root of the basal diameter as dependent variables. Isolation index does not meet this standard of homoscedasticity and was analysed by nonparametric test (SOKAL & ROHLF, 1984). We selected for the analysis only squares having at least one plant with a nest of L. niger.
Results
The correlation test (n = 4163, p < 0.05) shows that the height of the plant is strongly correlated (r = 0.71) with D1_halim and this in turn with D2_halim (r = 0.91). Therefore we decided to reject D1_halim from the analyses.
LOCAL SCALE. HIGH DENSITY AREAS.
Lasius niger plant selection.
Along the two 50 m transects 70 of the 212 plants (33%) checked had an ant nest at the base (70 ant nests in 100 m). Plants with L. niger were significantly higher, had bigger aerial parts (bigger D2_halim) and basal diameter than plants without ants (Wilks lambda = 0.92, F 3,198 = 6.07, p < 0.0001). The ratio of height / D1_halim (plant shape) was significantly greater in the plants without ants, that is to say, the ant selects wider rather than higher plants, that is, comparatively scrubby ones (Mann Whitney U, Z = -2.27, p < 0.05). The results of analysis of variance are shown in Table 1.
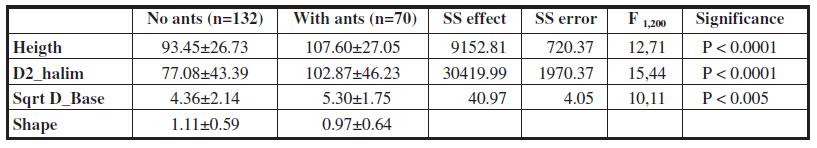
In addition, there were significant differences in the degree of isolation between plants with and without ant nests, the plants with ants being more isolated (mean value of isolation: 2.06 ± 1.08) than unoccupied ones (average value of class of isolation 1.69 ± 1.16). (Mann Whitney U, Z = -2.06, p < 0.05).
Plebejus argus plant selection.
Fifty percent of the plants analysed in the transects had P. argus eggs. We also found plants with eggs but without ants (n = 40) and plants without eggs but with ants (n = 9). The probability of finding P. argus eggs in plants with Lasius is 60.3% and in plants without ants is 39.6%, being this difference significant (X2: 59.11, df = 1, p = 0.0001).
Regardless of the presence of ants, plants used by P. argus for oviposition followed the same pattern as those selected by ants, choosing higher plants with bigger diameter 2 and basal diameter (Wilks lambda = 0.85, F 3,198 = 11.65 p < 0.001) (Table 2) the differences between plants with and without eggs being even higher than those with or without ants (see Table 1).
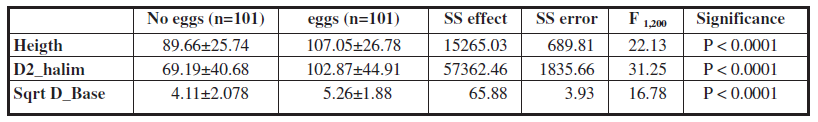
However, although there were no significant differences in the degree of isolation of plants (Mann Whitney U. Z = -1.27 p < 0.1982), plant shape was different (Mann Whitney U. Z = 3.75 p < 0. 0001) those with eggs having a lower width/height, ratio that is, a grater development of width in relation to height.
The results of the factor analysis show that plant architecture variables have a significant effect on both the presence of eggs (Wilks test 0.91 F 1.196 = 6.58 p < 0.0001) and the combined effect with L. niger (Wilks test 0.94, F 1.196 = 3.86 p < 0.05) (no intercept model overparameterized Type III). L. niger factor is not significant (Wilks test 0.98 F 1.196 = 1.57 ns).
Plants without ants and without P. argus eggs were shorter and with a lower basal diameter than plants with eggs, both with ants and without them (Fig. 1). It is striking that plants without ants but with eggs have a bigger basal diameter and are taller than plants without ants or eggs (Unequal N HSD post hoc test Fig. 1). Those plants are attractive to butterflies because of their larger size or even for having nests of L. niger until recent times and therefore can still maintain some odorous signals.
There are no significant differences between plants with eggs and no ants and plants with eggs and ants (Unequal N HSD post hoc test Fig. 1). These plants are higher and with bigger diameters than plants without eggs. The low number of plants with ants and without eggs (n = 9) is not enough to allow a proper assessment of this category. The high variability present in all measurements does not allow us to obtain conclusive results on this issue.
LANDSCAPE SCALE. LOW AND HIGH DENSITY AREAS
In the fifty 1 ha squares examined across the DNP we measured a total of 4,163 plants, 616 of them having L. niger ants at the base (14.8%). At this scale there are still significant differences in plants according to the presence of ants, which again are taller, with bigger diameter 2 and bigger basal diameter (Wilks lambda = 0.97 F 3.4159 = 39.79 p < 0.0001). That is, plants with ants, were again more scrubby than those without them.
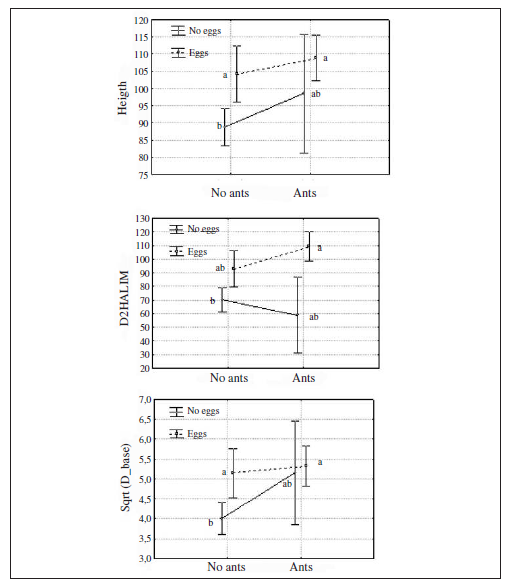
The 50 squares checked for ants have a distribution of frequencies of occupied plants skewed to the left (Fig. 2), with an average of 0.24 ± 0.22 of occupied plants and a median of 0.1923 (maximum 0.85 and minimum 0.009). Based on the latter value we categorized the squares as high density (0.2 and above), low density (0 to 0.2) or without ants (0). In squares with no ants we counted 0.17 ± 0.07 (mean ± SE) butterflies while in UTM squares categorised as low density ones we counted 7.31 ± 2.29 (mean ± SE) butterflies. This low density of butterfly adults made eggs hard to find on plants and consequently we concentrated the analysis of butterfly preferences only on high density areas.
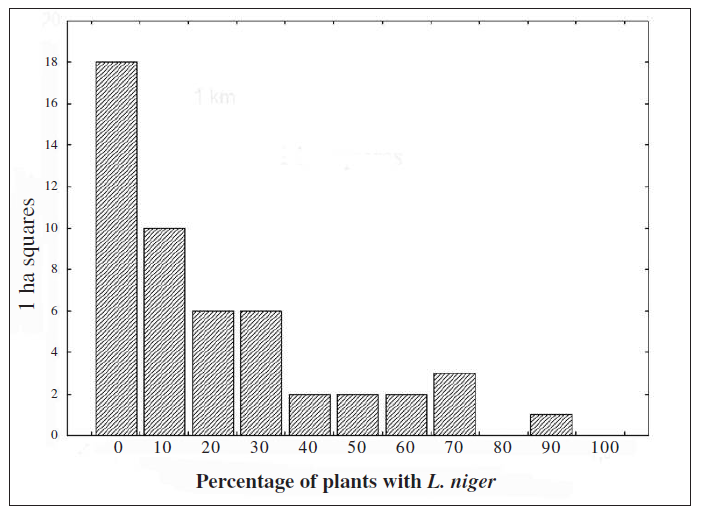
In squares with no ants (zero density) plants were smaller (mean ± SD, 65.46 ± 35.73) and with a lower basal diameter (mean ± SD, 12.46 ± 16.72) than in the other two density categories. Low density squares had plants with a mean height of 76.09 ± 35.64 (SD) and basal diameter of 14.95 ± 18.64 (SD) while high density squares had plants with a mean height of 71.18 ± 34.38 (SD) and basal diameter of 17.27 ± 19.78 (SD) (Wilks lambda = 0.18 F6.8318 = 1894.0 p < 0.0001).
In the other two density categories, both the presence of L. niger (Wilks lambda = 0.98 F 2.2568 = 26.17. p < 0.0001), the density category (Wilks lambda = 0.98 F 2.2568 = 26.62 p < 0.0001) and the combined effect of both factors (Wilks lambda = 0.99 F 2.2568 = 3.53 p < 0.05) had a significant effect on plant characteristics (significant multifactorial Anova type III decomposition).
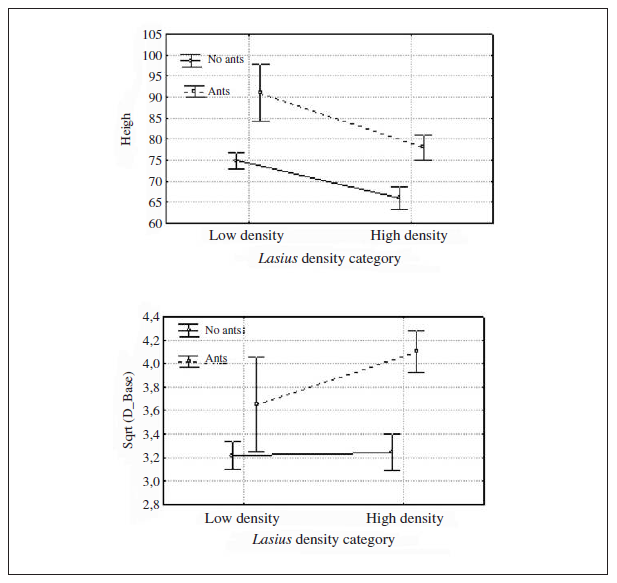
The height and diameter 2 in plants with L. niger were significantly different in squares with low occupation density (Fig. 3). These plants were taller and with a greater diameter 2 in this low density category, while basal diameter had the greatest value in plants with Lasius in high density areas.
Discussion
The physical support of ant nests is vital in the case of ants that live in extremely dry and adverse locations. The nature of sandy soil in the studied area becomes the limiting factor for building ant nests. L. niger, as many other species of ants, uses the roots of the plants as support for building their nests (GUTIÉRREZ et al., 2005). The aerial parts of the plant, provide a nest with a more favourable microclimate of humidity and temperature. Furthermore, daily and annual fluctuations of these factors tend to be even more buffered at shallow depth below the sand surface.
L. niger chooses plants with a greater aerial volume and a larger basal diameter. Furthermore, these plants are relatively “scrubby” and less isolated, which suggests a selection of particular plants that ensure a broad and durable physical support to ants in a sandy soil. It is also important to note that these selected large plants must be among the oldest in the area and have been “available” for a longer time for colonisation. Indirectly, the size of these plants indicates that they are in areas that have not been recently cleared. In addition, the plants selected by ants are in turn less isolated. Ants prefer areas of dense vegetation, where microclimatic conditions allow foraging during summer time when high soil temperature limits ant activity (DOBLAS-MIRANDA & REYES-LÓPEZ, 2008), especially in the pioneering stages of succession to which this ant species is often linked (BOOMSMA & DE VRIES, 1980;BOOMSMA & VAN LOON, 1982). Furthermore, Lasius frequently feeds on invertebrates, often found in fragments of vegetation where they, in turn take shelter from high temperatures (HOLEC et al., 2006). The degree of isolation may also be related to the use of various plants as support in case of oversized nests that occupy larger areas.
An ovipositing behaviour conditioned by ants has been found in numerous species of butterflies with a myrmecophylic relationship (PIERCE & EDGAR, 1985;PIERCE & EASTEL, 1986; RODRÍGUEZ et al., 1991;PIERCE et al., 2002;PATRICELLI et al., 2011;JANSEN et al., 2012). About 75% of Lycaenidae species associate with ants and this association implies specific adaptations of larvae such as the development of ant-associated organs, suppression of ant aggressiveness via specific sounds or mimicking of the pheromones of ant brood (PIERCE et al., 2002; ÁLVAREZ et al., 2005). Finally, Lycaenidae species whose larvae or pupae live within ant nests for part of their life cycle use ants as oviposition clues (PIERCE & EDGAR, 1985; PIERCE et al., 2002).
In the case of P. argus, larvae usually stay during the day sheltered inside ant nests at the base of the plant, protected from high temperatures and predators, beginning to climb the plant for feeding at nightfall (RODRÍGUEZ et al., 1991). This behaviour may have evolved in response to the nocturnal foraging habit of Lasius, since ants seem to develop an individual behavioural response to temperature and luminosity fluctuations (DEPICKÉRE et al. 2008) with different periodic cycles of activity throughout the day that allow foraging in different parts of the habitat at different times of the day or night (BALLARD & PRUESS, 1980). Unlike other species that forage during daytime with high temperatures (CERDÁ & RETANA, 2000)L. niger is mainly a nocturnal forager during the summer, when temperatures in the study area at ground level may reach 60 C (unpublished data). In the DNP, L. niger presents activity peaks during the hours when temperatures are milder, sundown in summer, noon in winter (unpublished data).
In some butterfly species aggregation of larvae on host plants are considered a visual stimuli for ovipositing females (PIERCE & EDGAR, 1985). However, in this P. argus population in southern Spain adults are more active during sunny hours whereas larval aggregations prefer feeding at night. Therefore female host plant selection cannot respond to larvae. Furthermore timing coincidence between adults and larvae along the life cycle is short, suggesting that other signals apart from larvae could make an individual plant attractive to females during their flying period. Gravid females have been observed touching the plants several times before landing on the leaf litter on the bush base someway evaluating the quality of plants for oviposition. As a result plants selected by gravid females coincide to a large extent with the type of plants used by the ants.
P. argus females lay their eggs preferably on plants with L. niger and therefore the morphology of the selected plants is adjusted to those selected by ants (greater height and basal diameter, “scrubby” and less isolated plants). However, the difference between the diameters of the plants with and without eggs is bigger than the diameters of plants with and without ants, suggesting that butterflies choose plants with a higher aerial part which, in principle, must guarantee greater availability of resources (leaves, buds and flowers) for larval development. This pattern is consistent both at the local and landscape scale.
Therefore, it appears that the butterfly responds not only to the presence of ants, but also evaluates visually the potential of the plant to feed its progeny. Given the high burden of caterpillars that has been observed (more than 200 on a single plant, unpublished data) a wrong choice could lead to defoliation of the plant and force a shift to neighbouring plants with the risks involved (ANTHES et al., 2003;LIU et al., 2006). In an observational study of movement, females have been observed performing short flights around a shrub touching the plant several times before landing on the base. The sequence followed should be first a visual evaluation of the plant and then a perception, of the presence of L. niger ants.
The foraging area in incipient ant nests is usually small and ants often leave little scent marks in their path. However, mature colonies can cover a large area and actively mark their tracks (MAILLEUX et al., 2003). There is also considerable individual variation in the frequency and use of marking (BECKERS et al., 1992). Therefore, plants with eggs but no ants could be plants adjacent to other plants with nest within their home range which therefore maintain their fragrant signals. By touching host plants with their antennae butterflies may even be able to recognise the chemical traces left by worker ants (PIERCE & EDGAR, 1985). A successful offspring on these plants will depend on the proximity to a nest of a neighbouring plant and the foraging frequency of ants on it. In these cases, the aerial parts are significantly larger than in others, which further strengthen the idea of selection of plants by different signals other than merely the existence of ants.
Zero density areas had the smallest plants, whereas plants in low density areas are the tallest, suggesting that areas with a recent colonisation by plants are rejected by both ants and butterflies. In those areas of low density of occupation, plants with Lasius are significantly higher than plants in the high density zones, that is, ants choose the bigger plants they can find in those low quality areas to start colonisation.
In the DNP clearing of old scrub areas is a valuable management tool that has been used since ancient times. This scrub clearing promotes the appearance of ephemeral grassland and favours shrub regeneration, increases diversity and primary production, and is a basic management measure that positively affects rabbit populations, depleted by viral hemorrhagic pneumonia (MORENO & VILLAFUERTES, 2005). However, actions such as mowing or scrub clearing in the habitat of butterflies have an effect on their populations and may require specific dampening measures (ANTHES et al., 2003; STRAUSZ, 2012; BERGSTRÖM, 2005;FARTMANN & TIMMERMANN, 2006;JOHST et al., 2006;EICHEL, S. & FARTMANN, 2008). In our study plant architecture provides a visual method for differentiating plants used by P. argus and L. niger, becoming a simple and easy management tool useful for decision making processes in clearing and cutting of scrub areas. Saving visually small stands of habitat during the process of scrub clearing, keeping plants with greater chance of being occupied by both ants and butterflies and maintaining small nuclei of them could greatly improve conservation of the plant itself and butterfly, accelerating recovery and colonisation.
BIBLIOGRAPHY
AGOSTA, S. J., 2006.– On ecological fitting, plant-insect associations, herbivore host shifts, and host plant selection.– Oikos, 114(3): 556-565.
ALBANO-ARAÚJO, A. A., D’ARC DE PAULA, J., ALVES-CARNEIRO, M. A. & SCHOEREDER, J. H., 2006.– Effects of host plant architecture on colonization by galling insects.– Austral Ecology, 31: 343-348.
ÁLVAREZ, M., RUIZ, E., LÓPEZ-MUNGUIRA, M., MARTÍNEZ, M. D., & HERNÁNDEZ, J. M., 2005.– La emisión de sonido en hormigas y mariposas: ¿un sistema de comunicación entre especies?- Actas de la XVI Bienal de la Real Sociedad Española de Historia Natural: 39-41.
ANTHES, N., FARTMANN, T., HERMANN, G. & KAULE, G., 2003.– Combining larval habitat quality and metapopulation structure - the key for successful management of pre-alpine Euphydryas aurinia colonies.– Journal of Insect Conservation, .: 175-185.
AWMACK, C. S. & LEATHER, S. R., 2002.– Host plant quality and fecundity in herbivorous insects.– Annual Review of Entomology, 47: 817-844.
BARROS-BELLANDA, C. H. & ZUCOLOTO, F. S., 2005.– Egg cannibalism in Ascia monuste in the field; opportunistic, preferential and very frequent.– Journal of Ethology, 23: 133-138 (doi 10.1007/s10164-004- 0138-y).
BECKERS, R., DENEUBOURG, J. L. & GOSS, S., 1992.– Trail laying behaviour during food recruitment in the ant Lasius niger (L.).– Insectes Sociaux, 39: 59-72.
BERGSTRÖM, A., 2005.– Oviposition site preferences of the threatened butterfly Parnassius mnemosyne - implications for conservation.– Journal of Insect Conservation, .: 21-27.
BEYER, L. J. & SCHULTZ, C. B., 2010.– Oviposition selection by a rare grass skipper Polites mardon in montane habitats: Advancing ecological understanding to develop conservation strategies.– Biological Conservation, 143(4): 862-872.
BOOMSMA, J. J. & DE VRIES, A., 1980.– Ant species distribution in a sandy coastal plain.– Ecological Entomology, .: 189-204.
BOOMSMA, J. J. & VAN LOON, A. J., 1982.– Structure and diversity of ant communities in successive coastal dune valleys.– Journal of Animal Ecology, 51: 957-974.
BOOMSMA, J. J. VAN DER LEE, G. A. & VAN DER HAVE, T. M., 1982.– On the production ecology of Lasius niger (Hymenoptera: Formicidae) in successive coastal dune valleys.– Journal of Animal Ecology, 51: 975- 991.
BRISTOW, C. M., 1991.– Why are so few aphids ant-tended? - In C. R. HUXLEY & D. F. CUTLER (eds). Ant- Plant Interactions: 104-119. Oxford University Press, Oxford.
BROWER, D. M. & STAMP, N. S., 1987.– Patterns of oviposition in Hemileuca lucina (Saturniidae).– Journal of the Lepidopterists’ Society, 41: 131-140.
CAMARGO, R. S., FORTI, L. C., DE MATOS, C. A. O. LOPES, J. F. & DE ANDRADE, A. P. P., 2003.– Physical resistance as a criterion in the selection of foraging material by Acromyrmex subterraneus brunneus Forel, 1911(Hym. Formicidae).– Journal of Applied Entomology, 128(5): 329-331.
CERDÁ, X. & RETANA, J., 2000.– Alternative strategies by thermophilic ants to cope with extreme heat: individual versus colony level traits.– Oikos, 89: 155-163.
CLARK, L. G. & DENNEHY, T. J., 1988.– Oviposition behaviour of grape berry moth.– Entomologica Experimentalis et Applicata, 47: 223-230.
COURTNEY, S. P. & COURTNEY, S., 1982.– The edge effect in butterfly oviposition: causality in Anthocharis cardamines and related species.– Ecological Entomology, .: 131-137.
COURTNEY, S. P., 1984.– The Evolution of egg clustering by butterflies and other insects.– American Naturalist, 123(2): 276-281.
DENNIS, R. R., 1983.– Pattern and response in egglaying of the orange tip butterfly Anthocharis cardamines (L.) (Pieridae).– Vasculum, 98: 27-43.
DENNIS, R. R., 1985.– The edge effect in butterfly oviposition, host plant condition, edge effect breakdown and opportunism.– Entomologist’s Gazette, 36: 285-291.
DEPICKÉRE, S., FRESNEAU, D., & DENEUBOURG, J. L., 2008.– Effect of social and environmental factors on ant aggregation: A general response? - Journal of Insect Physiology, 54: 1349-1355.
DOAK, P., KAREIVA, P. & KINGSOLVER, J., 2006.– Fitness consequences of choosy oviposition for a timelimited butterfly.– Ecology, 87: 395-408.
DOBLAS-MIRANDA, E. & REYES-LÓPEZ, J., 2008.– Foraging strategy quick response to temperature of Messor barbarus (Hymenoptera: Formicidae) in Mediterranean environments.– Environmental Entomology, 37(4): 857-61.
EICHEL, S. & FARTMANN, T., 2008.– Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation and patch area.– Journal of Insect Conservation, 12: 677-688.
FARTMANN, T. & TIMMERMANN, K., 2006.– Where to find the eggs and how to manage the breeding sites of the Brown Hairstreak (Thecla betulae (Linnaeus, 1758) in Central Europe? - Nota lepidopterologica, 29(1/2): 117-126.
FERNÁNDEZ-HAEGER, J., GARCÍA-GARCÍA, I. & AMAT, J. A., 1976.– Guía de las Mariposas de Doñana.– Naturalia Hispanica, .: 1-55.
FOURCASSIÉ, V., SCHMITT, T., & DETRAIN, C., 2012.– Impact of interference competition on exploration and food exploitation in the ant Lasius niger.– Psyche, 2012: doi:10.1155/2012/383757.
GARCÍA-NOVO, F., MERINO, J., RAMÍREZ-DIAZ, L., RODENAS, M., SANCHO, F., TORRES, A., GONZÁLEZ-BERNÁLDEZ, F., DÍAZ-PINEDA, F., ALLIER, C., BRESSET, V. & LACOSTE, A., 1977.– Doñana. Prospección e inventario de ecosistemas.– Monografías ICONA, 18: 244 pp. Ministerio de Agricultura, Madrid.
GONÇALVES-ALVIM, S. J. & FERNANDES, G. W., 2001.– Biodiversity of galling insects: historical, community and habitat effects in four Neotropical savannas.– Biodiversity Conservation, 10: 70-98.
GUTIÉRREZ, D., FERNÁNDEZ, P., SEYMOUR, A. & JORDANO, D., 2005.– Habitat distribution models: are mutualist distributions good predictors of their associates? - Ecological Applications, 15(1): 3-18.
HOLEC, M., FROUZ, J. & POKOMY, R., 2006.– The influence of different vegetation patches on the spatial distribution of nests and the epigeic activity of ants (Lasius niger) on a spoil dump after brown coal mining (Czech Republic).– European Journal of Soil Biology, 42(3): 158-165.
JANSEN, S. H. D. R., HOLMGREN, M., VAN LANGEVELDE, F. & WYNHOFF, I., 2012.– Resource use of specialist butterflies in agricultural landscapes: conservation lessons from the butterfly Phengaris (Maculinea) nausithous.– Journal of Insect Conservation, 16: 921-930 (doi 10.1007/s10841-012-9479-y).
JOHST, K., DRECHSLER, M., THOMAS, J. & SETTELE, J., 2006.– Influence of mowing on the persistence of two endangered large blue butterfly species.– Journal of Applied Ecology, 43: 333-342.
JORDANO, D., RODRÍGUEZ, J., THOMAS, C. D. & FERNÁNDEZ-HAEGER, J., 1992.– The distribution and density of a lycaenid butterfly in relation to Lasius ants.– Oecologia, 91: 439-446.
LIU, W., WANG, Y. & XU, R., 2006.– Habitat utilization by ovipositing females and larvae of the Marsh fritillary (Euphydryas aurinia) in a mosaic of meadows and croplands.– Journal of Insect Conservation, 10: 351-360 (doi 10.1007/s10841-006-9009-x).
LÖRTSCHER, M., ERHARDT, A. & ZETTEL, J., 1995.– Microdistribution of butterflies in a mosaic-like habitat: the role of nectar sources.– Ecography, 18: 15-26.
MAILLEUX, A. C., DENEUBOURG, J. L. & DETRAIN, C., 2003.– How does colony growth influence communication in ants? - Insectes Sociaux, 50: 24-31.
MARTIN-STRAUSZ, M., FIEDLER, K., FRANZE´N, M. & WIEMERS, M., 2012.– Habitat and host plant use of the Large Copper Butterfly Lycaena dispar in an urban environment.– Journal of Insect Conservation, 16: 709-721.
MORENO, S. & VILLAFUERTE, R., 1995.– Traditional management of scrubland for the conservation of rabbits Oryctolagus cuniculus and their predators in Doñana National Park. Spain.– Biological Conservation, 73: 81- 85.
OBREGÓN, R., DE HARO, S., JORDANO, D. & FERNÁNDEZ-HAEGER, J., 2012.– Lampides boeticus (Lepidoptera: Lycaenidae) preys on cocoons of its own specific parasitoid Cotesia specularis (Hymenoptera: Braconidae).– Journal of Insect Behavior, 25: 514-517 (doi 10.1007/s10905-012-9318-8).
PATRICELLI, D., BARBERO, F., LA MORGIA, V., CASACCI, L. P., WITEK, M., BALLETTO, E. & BONELLI, S., 2011.– To lay or not to lay: oviposition of Maculinea arion in relation to Myrmica ant presence and host plant phenology.– Animal Behaviour, 82: 791-799.
PIERCE, N. E. & EASTEL, S., 1986.– The selective advantage of attendant ants for the larvae of a lycaenid butterfly, Glaucopsyche lygdamus.– Journal of Animal Ecology, 55: 451-462.
PIERCE, N. E. & ELGAR, M. A., 1985.– The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous lycaenid butterfly.– Behavioral Ecology and Sociobiology, 16: 209-222.
PIERCE, N. E., BRABY, M. F., HEATH, A., LOHMAN, D. J., MATHEW, J. RAND, D. B. & TRAVASSOS, M. A., 2002.– The ecology and evolution of ant association in the Lycaenidae (Lepidoptera).– Annual Review of Entomology, 47: 733-71.
PRADEL, K. & FISCHER, K., 2011.– Living on the edge: Habitat and host-plant selection in the butterfly Lycaena tityrus (Lepidoptera: Lycaenidae) close to its northern range limit.– Journal of Research on the Lepidoptera, 44: 35-41.
RABASA, S. G., GUTIÉRREZ, D. & ESCUDERO, A., 2004.– Egg laying by a butterfly on a fragmented host plant: a multi-level approach.– Ecography, 28: 629-639.
REID, A. M. & HOCHULI, D. F., 2007.– Grassland invertebrate assemblages in managed landscapes: Effect of host plant and microhabitat architecture.– Austral Ecology, 32: 708-718.
REUDLER-TALSMA, J. H., BIERE, A., HARVEY, J. A. & VAN NOUHUYS, S., 2008.– Oviposition cues for a specialist butterfly-plant chemistry and size.– Journal of Chemical Ecology, 34: 1202-1212.
RIIHIMÄKI, J., VEHVILÄINEN, H., KAITANIEMI, P. & KORICHEVA, J., 2006.– Host tree architecture mediates the effect of predators on herbivore survival.– Ecological Entomology, 31(3): 227-235.
RIIHIMÄKI, J., VEHVILÄINEN, H., KAITANIEMI, P. & KORICHEVA, J., 2006.– Host tree architecture mediates the effect of predators on herbivore survival.– Ecological Entomology, 31(3): 227-235.
RODRÍGUEZ, J., FERNÁNDEZ-HAEGER, J. & JORDANO, D., 1991.– El ciclo biológico de Plebejus argus (Linnaeus, 1758) en el Parque Nacional de Doñana (SW de España).– SHILAP Revista de lepidopterología, 19(76): 241-252.
RUDGERS, J. & WHITNEY, K. D., 2006.– Interactions between insect herbivores and a plant architectural dimorphism.– Journal of Ecology, 94: 1249-1260.
SAKATA, H. & HASHIMOTO, Y., 2000.– Should aphids attract or repel ants? Effect of rival aphids and extrafloral nectaries on ant-aphid interactions.– Population Ecology, 42: 171-178.
SCHOONHOVEN, L. M., BEERLING, E. A. M., BRAAKSMAN, R. & VAN VUGT, Y., 1990.– Does the imported cabbageworm Pieris rapae use an oviposition deterring pheromone? - Journal of Chemical Ecology, 16(5): 1649-1655.
SHANSHAN, Z., YILI, J., JIANJUN, T. & XIN, C., 2009.– The invasive plant Solidago canadensis L. suppresses local soil pathogens through allelopathy.– Applied Soil Ecology, 41: 215-222.
SOKAL, R. R. & ROHLF, F. J., 1984.– Introducción a la bioestadística: 362 pp. Editorial Reverté, Barcelona.
SUWARNO, CHE SALMAH, M. R., ALI, A. & HASSAN, A. A., 2010.– Oviposition preference of swallowtail butterfly, Papilio polytes (Lepidoptera: Papilionidae) on four Rutaceae (Sapindales) host plant species.– Insect Science, 17: 369-378.
THOMAS, J. A. SIMCOX, D. J., CLARKE, R. T., 2009.– Successful conservation of a threatened Maculinea butterfly.– Science, 325: 80-83.
THOMPSON, J. N. & PELLMYR, O., 1991.– Evolution of oviposition behavior and host preference in Lepidoptera.– Annual Review of Entomology, 36: 65-89.
WAGNER, D. & KURINA, L., 1997.– The influence of ants and water availability on oviposition behaviour and survivorship of a facultatively ant-tended herbivore.– Ecological Entomology, 22: 352-360.
WYNHOFF, I., GRUTTERS, M. & VAN LANGEVELDE, F., 2008.– Looking for the ants: selection of oviposition sites by two myrmecophilous butterfly species.– Animal Biology, 58: 371-388.
Notas de autor