

Xylomoia strix Mikkola, 1980 in Poland with comments on its biology and ecology (Lepidoptera: Noctuidae)
Xylomoia strix Mikkola, 1980 en Polonia con comentarios sobre su biología y ecología (Lepidoptera: Noctuidae)
Xylomoia strix Mikkola, 1980 in Poland with comments on its biology and ecology (Lepidoptera: Noctuidae)
SHILAP Revista de lepidopterología, vol. 44, núm. 174, 2016
Sociedad Hispano-Luso-Americana de Lepidopterología
Recepción: 18 Marzo 2015
Aprobación: 18 Mayo 2015
Abstract: The distribution of Xylomoia strix Mikkola, 1980 at a number of new localities in eastern Poland is described. The species’ biology and ecological requirements are also given.
Keywords: Lepidoptera, Noctuidae, Xylomoia strix, distribution, biology, ecology, Poland.
Resumen: Se describe la distribución de Xylomoia strix Mikkola, 1980 en algunas nuevas localidades del este de Polonia. También se incluye la biología de la especie y sus requerimientos ecológicos.
Palabras clave: Lepidoptera, Noctuidae, Xylomoia strix, distribución, biología, ecología, Polonia.
Introduction
The genus Xylomoia Staudinger, 1892 is represented worldwide by 7 species, one of which is known from North America; the other 6 are exclusive to the Palaearctic. To date 4 species have been found in Europe, all within the last 35 years: X. strix Mikkola, 1980 (MIKKOLA, 1980), X. graminea (Graeser, [1889] 1888) (NOWACKI, 1989), as well as X. retinax Mikkola, 1998 and X. stangelmaieri Mikkola, 1998 (MIKKOLA, 1998). Characteristic of these Xylomoia species is the fact that their distribution as well as the biology and ecology of the particular species are poorly known. Among the European species only X. graminea has a fairly large number of known localities, mainly in Poland, where it is often numerous (NOWACKI, & PALKA, 2013). Beyond Poland it is known from only single localities in Ukraine and Russia (ZILLI et al., 2005). The other species found in Europe are very similar to one another in external appearance. In practice, they form a uniform group of sibling species “X. strix”, the status of which will be eventually explained once their biology has been determined and genetic studies carried out. Worth stressing is the fact that these species are exceptionally rare, which does not make studying them any easier. X. strix (Fig. 7) has so far been recorded in Estonia, Finland, Latvia, Poland, Russia and Ukraine. It is from this last-mentioned country that the oldest specimen of the species comes: it was caught in the Kaniewo reserve in 1960 (MIKKOLA, 1998,ZILLI et al., 2005). In Poland X. strix was first recorded 20 years ago at Zawadówka near Chelm (NOWACKI & SEKULA, 1994, BUSZKO et al., 1996), but in recent years it has not been found again in this locality. In 2013 a new locality was discovered in the valley of the River Bug near Skryhiczyn (NOWACKI & PALKA, 2014). The discovery of this locality provided confirmation of the occurrence of X. strix in Poland. This is of great significance because the species is an EU priority species, listed in Annexes II and IV of the Habitats Directive. In Poland, too, it is a legally protected species. As a result of longterm studies of the distribution of lepidopterans in Poland the rarest species have been placed on “The Red List of Vanishing and Threatened Animals” (BUSZKO & NOWACKI, 2002). In this list X. strix has been categorised as DD, i.e. among species rare at the European scale, occurring in isolated localities, whose biology and ecology are often unknown. More than 10 years ago, the authors of this list stated categorically that there was an urgent need to study the biology, ecology and distribution of this noctuid. The larval host plant of X. strix was recently found to be Equisetum hyemale L. (AHOLA & SILVONEN, 2008). Armed with this knowledge, we were able to undertake a study of the distribution, biology and ecology of this moth in Poland.
Material and methods
The aim of the study undertaken in 2013 and 2014 was to get to know the current distribution of X. strix in Poland and to discover its biology and ecology. The following methods were applied:
To determine the species’ distribution, field work was carried out at 19 localities in eastern Poland where suitably large patches of the host plant – rough horsetail (E. hyemale) - were growing. Establishing these localities was no easy task, because E. hyemale occurs very locally, usually in more or less dense patches from a few tens to several hundred m2 in area. As this plant is not a protected species, there is no precise knowledge of its distribution in Poland. The assistance of botanists and foresters was thus invaluable in the search for suitable patches. The localities where the fieldwork was carried out were as follows: Biebrza National Park: Bialogra˛dy (UTM) FE03, Osowiec Twierdza FE02; Bialowiez . a National Park: Hajnówka FD74; Siemiatycze FD20, Siemiatycze Station FD30; “Urle” Nature Reserve near Z . elizno FC34; Parczew Forests: Sowin FC31; Zarzecz Lukowski FC05; Bochotnica near Kazimierz Dolny EB78; Kazimierzów EB67; Nowe Komaszyce EB76; Stalowa Wola EB70; Kurylówka FA07; Radruz . near Horyniec Zdrój FA66; Zwierzyniec Bialy Slup FB40; Malice FB91; S´ lipcze KS82; De˛bowiec near Dubienka GB05; Zawadówka near Chelm FB66; Orchówek near Wlodawa FC81; Rozwadówka FC53. These localities were monitored for the presence of X. strix. In all of them signs of larval foraging on the stems of E. hyemale were looked for in April-May and again in August-September. In some localities adult moths were caught at light during their flight period. This was done using light traps equipped with 20 W UV fluorescent tubes and with 500 W generator-powered mercury vapour lamps placed in front of a white screen. The light traps and screen + lamp arrangements were always situated in patches of rough horsetail (E. hyemale).
In order to establish the biology and ecological requirements of X. strix regular observations of the species’ development were carried out at the natural localities at Malice and Radruz . from the beginning of April until the end of October. In addition, moths were bred on their host plants placed in gauze incubators under natural conditions. These observations enabled the dates of appearance of the various developmental stages and of the onset and site of larval hibernation to be determined.
X. strix is legally protected in Poland, so for this research special permission had to be sought in order for the usual prohibitions regarding protected species to be waived. The relevant permits were granted by the General Director for Environmental Conservation and the Regional Directors for Environmental Conservation in Lublin and Poznan´. We would like to express our gratitude to everyone who assisted us during this research, in particular Messrs. Krzysztof Fra˛ckiel, Bogdan Jaroszewicz, Marek Kucharczyk, Bogdan Lorenc, Grzegorz Szafran, Dariusz Wasiluk and Janusz Wójciak.
Results
DISTRIBUTION
The fieldwork showed X. strix to be present at 6 localities in eastern Poland, from the south eastern part of western Polesie, through Wolyn´ Polesie, the eastern part of the Lublin Upland, the Wolyn´ Upland to the central and eastern Roztocze (Fig. 1). These localities are as follows:
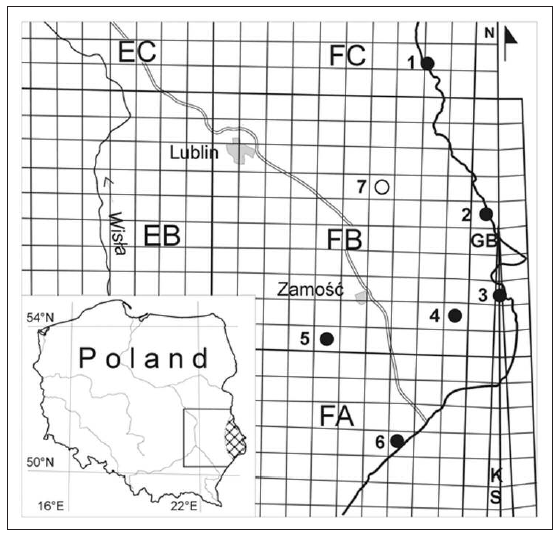
This locality is in the Bug valley, along a railway embankment running by a patch of riparian woodland. Rough horsetail (E. hyemale) grows abundantly on the slopes of the embankment and inside the riparian woodland.
This locality of X. strix extends for a distance of ca 70 m along the River Bug, some 30 m from the water, in a degraded but regenerating riparian woodland with a large proportion of hazel. There are many dense patches of rough horsetail (E. hyemale), some of them on damp ground.
This locality of X. strix extends for a distance of ca 70 m along the River Bug, some 50 m from the water, in a degraded elm-ash riparian woodland with a large proportion of hazel scrub. The rough horsetail (E. hyemale) plants in the ground layer are in good condition and form very dense patches.
This locality lies in the valley of the River Huczwa, in a lime-oak-hornbeam forest; the trees are of various ages. The ground layer is unevenly shaded, and this has favoured the growth of extensive patches of well-developed rough horsetail (E. hyemale).
This locality is a patch of rough horsetail by the road from Zwierzyniec to Józefów Roztoczan´ski, about 1 km before the level crossing on the Zwierzyniec side. The patch lies a short distance from the S´ wierszcz, a minor tributary of the River Wieprz. The 6 m wide patch of rough horsetail extends along the roadside for a distance of 80 m. Single plants have also been found in the forest on both sides of the road, including a forest that lies within the boundaries of the Roztocze National Park.
This locality lies along a small stream, the Radruz . ka, which is a tributary of the River Smolinka. It is in a lime-oak-hornbeam woodland where the trees are of various ages. The considerable proportion of underbrush strongly shades the ground layer, hence the E. hyemale plants over an extensive area grow in a compact swathe. They are very delicate, however, a condition that impedes their colonisation by X. strix caterpillars.
HABITAT PREFERENCES
The results of the fieldwork show that X. strix localities are always patches of rough horsetail (E. hyemale) growing in woodland ecosystems, often in the neighbourhood of roads and railway lines. The species typically occurs in riparian and lime-oak-hornbeam woodlands. All the woodland ecosystems where X. strix was found were situated on gentle slopes of valleys, or in the immediate vicinity of rivers or streams. The soils characteristic of these sites are readily permeable to water, which flows away down the slopes. Peat bogs, where stagnant ground water lies just below the surface, were never colonised by X. strix. The most suitable ecosystem was lime-oak-hornbeam woodland, which supported large populations of this moth. At Malice this ecosystem is natural with dominant lime, oak and hornbeam of various ages; the trees here shade the forest floor unevenly. This favours the growth of extensive rough horsetail (E. hyemale) patches of excellent quality (Fig. 3). In this locality rough horsetail forms a swathe of perfectly developed plants of suitable diameter; in places it forms single clumps, but the plants are still in such good condition that caterpillars of X. strix can complete their development there. It is very important that the horsetail plants are of the right thickness, otherwise the caterpillars of X. strix cannot pupate within them. In contrast, the situation at Radruz . in the Nowiny Horynieckie Forestry District is quite different. Here, X. strix is found in a oak-hornbeam tree stand consisting of a number of different species of various ages. But there is a considerable underbrush of young trees and shrubs, which throws a strong shade on to the ground layer. Hence, although there is an extensive, compact swathe of rough horsetail growing there, the plants are very delicate: their diameters are too small to be colonised by caterpillars, and for pupation they are totally unsuitable. Only in a few spots, where trees had been felled, was the forest floor sufficiently well illuminated to permit the horsetail plants to grow to a diameter facilitating the development of X. strix caterpillars.
Riparian forest is the second type of woodland ecosystem in which X. strix was found. There are ecosystems of this type at the De˛bowiec, Orchówek and S´lipcze localities. At each the riparian forest was of a slightly different character, but common to all were patches of rough horsetail (E. hyemale) in the ground layer. In this ecosystem, as in the oak-hornbeam woodland, the key aspect affecting the condition of the horsetail plants, and hence the chances of development of X. strix, was the density of the tree canopy. Where the tree stand was young, in a degraded elm-ash riparian forest with a large proportion of hazel shrubs, making for a scrubby habitat, rough horsetail grew very densely, but the stems were too thin to be colonised by X. strix. In contrast, in the same ecosystem in open areas along a road, the horsetails grow abundantly, the plants are very robust and in good condition, and thus caterpillars of X. strix at such sites are plentiful (Fig. 4).
LIFE CYCLE
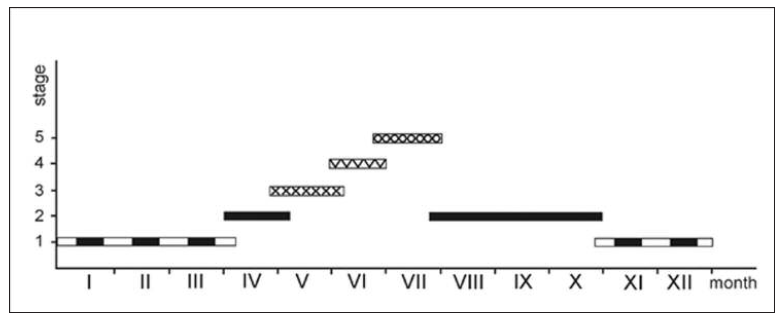
Observations of X. strix under natural conditions and while being bred in isolators showed that in Poland this species has one generation per year (Fig. 2). In spring (late March- early April) the overwintering caterpillars become active and resume feeding inside the stems of E. hyemale. Only one caterpillar feeds in each stem, and as it feeds it moves up the stem along successive internodes, cutting through the nodes. By the end of their development the caterpillars are foraging half-way up the plant and from the inside eat away all the pith but leave the epidermis intact. This part of the stem is completely translucent and often the caterpillar inside is visible (Fig. 5). In late April the fully-grown caterpillars leave the foraging site and look for somewhere to pupate. Some of them bite their way into a new stem through a hole at the lower end of an internode. The pith is eaten and the lower part of the internode is filled with frass, above which pupation takes place. Earlier, prior to pupation, in the upper part of the internode just below the node, the caterpillar cuts out an opening as a window partially bitten through from the upper side - this is the escape route for the imago (Fig. 6). Some caterpillars pupate in dead stems lying on the ground. Pupation takes place in late April – early May. In our breeding experiments the first caterpillar pupated on 26 April, and the last one on 9 May. The young pupa is olive-brown in colour, later gradually turning brown - first light brown and finally dark brown (Fig. 8). Two or three days before eclosion, the colour of the pupa intensifies, a sign that eclosion is imminent. Pupal development takes about one month, so that the adult moths appear in early June (Fig. 7). Eclosion from pupae taken from natural ecosystems took place on 2 June. The flight time of the adult moths is from early June to early July, with numbers peaking between 10 and 20 June. Light trapping during this period yielded over a dozen each night, regardless of the weather. In the second half of June fertilised females lay clutches of a few eggs each on rough horsetail stems. In late July the caterpillars hatch and begin feeding inside the E. hyemale stems. First- and second-instar caterpillars mostly feed half-way up the plant and higher up, and only in one internode. Significantly, foraging in most cases is communal: three, four and even six caterpillars were seen feeding in one internode. It happens that on one stem young caterpillars are simultaneously feeding in two different internodes. As a result of this feeding behaviours the internodes are completely destroyed and almost filled with frass; the young caterpillars then leave these internodes as they are incapable of biting their way through to the next internode. Caterpillars older than third instars feed singly. The caterpillar (Fig. 9) eats into the stem where it feeds in several internodes, cutting through the nodes between them. While feeding, caterpillars can change stems. In favourable weather or during a warm autumn, which was the case in 2013 and 2014, feeding can continue even until the end of October. In late October the fourth-instar caterpillars leave the stems in which they were feeding and search for a safe place to hibernate. They find this in dead, dry rough horsetail stems, usually lying on the ground among clumps of this plant.

Discussion
This study has shown that X. strix in Poland is found only within a compact area in the eastern part of the country, from south-eastern Polesie through the eastern part of the Lublin Upland, the Wolyn´ Upland to the central and eastern Roztocze. Within this area it is found at isolated localities with a similar type of ecosystem. In all cases these were riparian or oak-hornbeam woodlands, situated in the valleys of small rivers, with an abundance of rough horsetail (E. hyemale) in the ground layer. This moth has been found in similar ecosystems in Finland and Latvia (AHOLA & SILVONEN, 2008). It needs to be stressed that X. strix was not found in the Sobowice nature reserve at Zawadówka near Chelm (Fig. 1), where the species was first found in Poland (NOWACKI SEKULA, 1994), despite the area being monitored many times prior to and during this study. It may well be that the population of X. strix in this locality was collected to extinction by collectors and dealers in Lepidoptera. In the last 20 years X. strix was trapped at light many times in this locality. Moreover, we found numerous signs during our study that single rough horsetail plants had been cut out. This could indicate that collectors were obtaining larval instars, especially as at the remaining, newly discovered localities there were no signs of such cutting.
In view of the ecological requirements of X. strix, and its biology and occurrence at six localities, one may conclude that these conditions are sufficient for ensuring the survival and development of the populations of this protected insect in Poland. However, the habitats and populations of X. strix were not in the same condition in all the localities. Only at Malice was the habitat assessed as being in a wholly suitable state. There, the habitat consists of a large, compact patch of rough horsetail growing in lime-oak-hornbeam woodland with a well-preserved tree stand of different ages; this is what ensures the proper development of the moth’s larval host plant. At the other localities the state of the habitat cannot be deemed entirely satisfactory. This is because many of the patches of rough horsetail there grow in the excessive shade thrown by hazel shrubs and young trees, and this impedes the colonisation of the horsetail plants by X. strix. The state of the habitat has a direct effect on the numbers of X. strix in the various localities. The most numerous populations were found at Malice, S´lipcze, De˛bowiec and Zwierzyniec, where the relative abundance index and the numbers of imagines caught at light was suitably high.
The results of this study indicate two significant types of threat that could endanger the populations of X. strix at these localities. The more important one is natural succession, which comes into play following the removal of old trees from woodland ecosystems: the patches of rough horsetail then become overgrown with shrubs and young trees. This causes shading of the ground layer as a result of which the condition of the horsetail plants deteriorates, and this in turn has an adverse effect on their colonisation by X. strix. This is happening to a greater or lesser extent in all the localities. This threat can be fairly easily counteracted by removing the underbrush from the areas affected, as has been done at S´ lipcze. There, the underbrush was removed from a strip of land on the state frontier along the River Bug, which promoted the colonisation of the rough horsetail by X. strix. This demonstrates unequivocally that the removal of dense underbrush from areas inhabited by X. strix is an important measure for the conservation of this species.
The other exceedingly significant source of danger to local populations inhabiting very small areas is, as in the case of X. strix, the trapping of moths by collectors and dealers in Lepidoptera. The seriousness of this threat in readily accessible localities is underlined by the extinction of X. strix from the locality in the Sobowice nature reserve (Zawadówka). Unfortunately, with respect to this species, the legally protected status of the nature reserve was not sufficient to protect the population of X. strix living there. This threat should therefore be taken into consideration when planning conservation measures since the mere status of a legally protected species is not enough to ensure survival.
BIBLIOGRAPHY
AHOLA, M. & SILVONEN, K., 2008.– Larvae of Northern European Noctuidae, .: 672 pp. Viestipaino Oy, Tampere.
BUSZKO, J., JUNNILAINEN, J., KAITILA, J-P., NOWACKI, J., NUPPONEN, K. & PALKA, K., 1997.– New and rare to the Polish fauna species of Lepidoptera recorded in south-eastern Poland.– Wiadomos´ci Entomologiczne, 15: 105-115.
BUSZKO, J. & NOWACKI, J., 2002.– Lepidoptera.– In Z. GLOWACIN´ SKI. Red List of Threatened Animals in Poland: 80-87. Institute of Nature Conservation, Kraków.
MIKKOLA, K., 1980.– Two new noctuid species from Northern Europe: Polia sabmeana n. sp. and Xylomoia strix n. sp. (Lepidoptera, Noctuidae: Hadeninae and Amphipyrinae).– Notulae Entomologicae, 60: 217-222.
MIKKOLA, K., 1998.– Revision of the genus Xylomoia Staudinger (Lepidoptera: Noctuidae), with descriptions of two new species.– Systematic Entomology, 23: 173-186.
NOWACKI, J., 1989.– Xylomoia graminea (Graeser, 1888) a noctuid-moth new to the fauna of Poland and Europe (Lep., Noctuidae).– Przegla˛d Zoologiczny, 33: 445-447.
NOWACKI, J. & PALKA, K. 2013.– Contribution to the knowledge of distribution of the noctuid moths (Lepidoptera: Noctuidae) of eastern Poland.– Wiadomos´ci Entomologiczne, 32: 139-146.
NOWACKI, J. & PALKA, K., 2014.– New record of Xylomoia strix Mikkola, 1980 (Lepidoptera: Noctuidae) in Poland. Wiadomos´ci Entomologiczne, 33: 38-41.
NOWACKI, J. & SEKULA, W., 1994.– Xylomoia strix Mikkola, 1980 - a noctuid moth (Lepidoptera: Noctuidae) new to the Polish fauna.– Wiadomos´ci Entomologiczne, 13: 195-196.
ZILLI, A., RONKAY, L. & FIBIGER, M., 2005.– Noctuidae Europaeae. Apameini, .: 323 pp. Entomological Press, Søro.
Notas de autor